Teaching the Principles of Clinical Research
NIH Clinical Center Course Reaches Thousands Around the World
CREDIT: PATRICIA PIRINGER, NIH CLINCAL CENTER
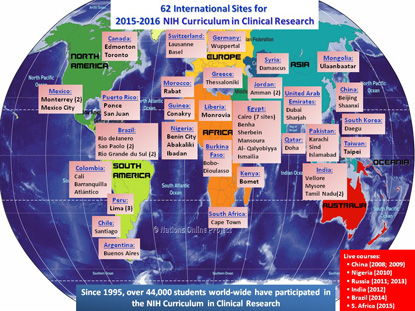
International Reach: The 2015–2016 “Introduction to the Principles and Practice of Clinical Research” course has 26 international participants: Argentina, Brazil, Burkina Faso, Canada, Chile, China, Colombia, Egypt, Greece, Guinea, India, Jordan, Kenya, Liberia, Mexico, Mongolia, Morocco, Nigeria, Pakistan, Peru, Qatar, South Korea, Switzerland, Syria, Taiwan, and the United Arab Emirates.
Celebrating its 20th anniversary, the course “Introduction to the Principles and Practice of Clinical Research” (IPPCR) has an impressive title and focuses on a clear goal: providing instruction on the basics of high-quality, safe, ethical, and efficiently conducted clinical research.
The course, one of the longest-running educational programs at the National Institutes of Health (NIH), started with a simple conversation in the early 1990s between then–NIH Director Harold E. Varmus and NIH Clinical Center Director John I. Gallin. Gallin had recently been appointed to his position and was assessing the strengths of the organization.
The Clinical Center has always excelled at collaboration and investigation, with 18 NIH institutes conducting their intramural studies at the hospital at that time. Varmus and Gallin knew that the Clinical Center was mastering this part of the research portfolio. What they discussed were other ways the Clinical Center could make an impact on the broader research community, specifically focusing on the fact that as a part of its overarching mission, the Clinical Center offered training opportunities for physicians, research fellows, and other clinical staff.
Gallin and Varmus agreed that the Clinical Center served as a model for clinical research. With such a leading role, the world’s largest hospital totally focused on clinical research was the perfect source for disseminating the best practices of clinical exploration. They determined that the best way to share this knowledge would be through a structured learning environment that drew upon the knowledge of the many talented biomedical scientists and clinical researchers working in the Clinical Center.
Gallin convened a group of experts from inside and outside the Clinical Center to establish the topics and curriculum that would create the foundation for “IPPCR.”
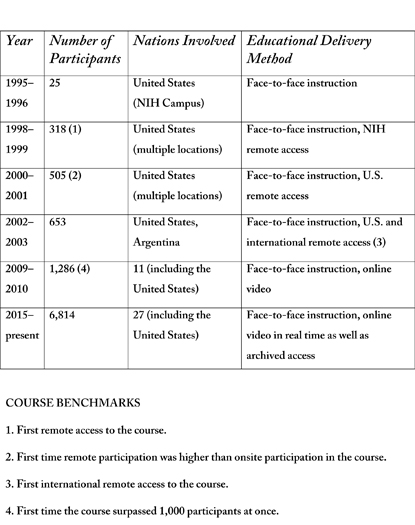
The “IPPCR” course was launched in the 1995–1996 academic year, with its first session held in September 1995 on the NIH campus. It included 25 participants.
With a premier course on clinical research established, the question was how to increase participation in the curriculum beyond the intramural program on NIH's Bethesda, Maryland, campus. NIH clinician-scientists are only a small portion of the national research community. And only a limited number of people could fit into a room on campus to learn from subject-matter experts. There had to be a way to make the class grow.
The solution was one that was fitting for a research hospital familiar with implementing innovations: using available technology to share the educational content remotely.
Within three years of the educational program’s launch in 1995, it offered remote access to NIH staff at the National Institute of Allergy and Infectious Diseases’ Rocky Mountain Lab in Hamilton, Montana, and the National Institute of Diabetes and Digestive and Kidney Diseases’ Epidemiology and Clinical Research Branch in Phoenix, Arizona.
Two years later, remote access was offered for Georgetown University (Washington, D.C.), the first non-NIH location to have access to “IPPCR.” In 2002, the first international remote access was provided through MAT and Asociados, based in Buenos Aries, Argentina. The international audience has been growing ever since.
Since the course’s inception, the number and content of the lectures have increased, as has course enrollment. To date, more than 28,000 individuals have enrolled in the “IPPCR” course. For the current academic year, there are nearly 7,000 students registered, with 524 at the NIH Bethesda campus and 6,290 at 123 remote academic sites. Fifty-four of the remote sites are outside of the United States, with twenty-six nations participating as partners for the current course. (See sidebar for full details.)
An abbreviated, weeklong version of the course has also been taught abroad to bring the face-to-face learning experience to clinicians overseas. Most recently, the course was taken to Cape Town, South Africa, in 2015 as a part of an agreement between NIH and the South African Medical Research Council. Other nations that have co-hosted the weeklong course with the NIH Clinical Center are China (Beijing and Chengdu), Russia, Nigeria, India, and Brazil.
In addition to teaching the principles of clinical research, the live international courses have also focused on enhancing collaborations between scientists in biomedical and behavioral research at NIH and those in the host nations. In addition, a core principle with all of the international partners has been to try to enable the participating students to become teachers themselves and to share their new knowledge with colleagues, following a “train the trainer” model.

The textbook used for the course, Principles and Practice of Clinical Research, was first edited by Gallin and published in 2002. Second and third editions were published in 2007 and 2012 and were edited by Gallin and Frederick P. Ognibene, the Clinical Center’s deputy director for Educational Affairs and Strategic Partnerships. As a testament to the international desire for clinical-research knowledge, the first edition of the text was translated into Japanese; the second edition into Chinese and Russian; and the third edition into Chinese and Japanese (in press). In addition, many academic institutions are using the textbook for other local instruction and programs in clinical research, in addition to “IPPCR.”
Recognizing that the clinical-research environment is constantly evolving, instructors have added topics to the class to ensure that its participants remain abreast of current areas of concern. Recent additions to the curriculum include management of clinical data and electronic health records; research on health disparities; and community-based research.
Since 2005, Ognibene has been “IPPCR” co-director with Gallin, and in 2013, Laura Lee Johnson, a biostatistician currently working at the FDA’s Center for Drug Evaluation and Research, joined as a third co-director.
In addition to “IPPCR,” the Clinical Center has two other courses in its core curriculum in clinical research. The “Principles of Clinical Pharmacology” course has been taught annually since 1998, and the “Ethical and Regulatory Aspects of Clinical Research” course since 1999. Both also use Web-based, long-distance learning techniques. For all three core curriculum courses, a cumulative total of 44,015 students have been enrolled since their inception.
“It has been incredibly gratifying to see this program grow from a concept into an international program,” said Gallin. “The hunger for this information, and the ability to draw upon internationally renowned experts, is a true testament to the influence and global reach of the Clinical Center.”
This page was last updated on Thursday, April 14, 2022
