Research Briefs
NEI, NCATS, NHGRI: RESEARCHERS DEVELOP FIRST STEM-CELL MODEL OF EYE DISEASE
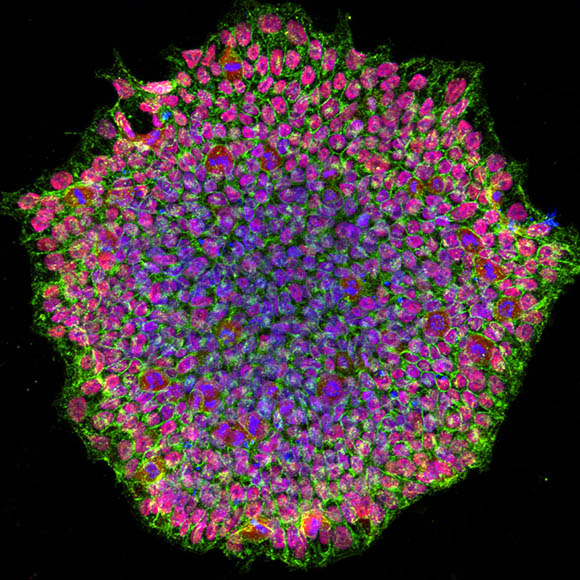
CREDIT: NEI
NEI et al.: A human induced pluripotent stem-cell colony from a patient with an eye disease called OCA. The image was acquired using a confocal microscope and is stained for pluripotency marker proteins. The red color depicts transcription factor OCT4, green is SSEA4 protein, and blue represents the nucleus of the cells.
NEI researchers have developed the first stem-cell model for oculocutaneous albinism (OCA), a set of genetic conditions that adversely affect pigmentation in the eye, skin, and hair. The disease-in-a-dish model will be used to further study this condition and to test new drug candidates.
People with OCA lack pigment in their retinal pigment epithelium (RPE), which supports the function of important light-sensing regions of the eye. This can result in a malformed optic nerve and an underdeveloped fovea, the part of the retina responsible for high-acuity vision. There is no approved treatment for most forms of OCA, and it cannot be studied very well using animal models.
In this method, published in Stem Cell Reports, researchers collected skin cells from patients with OCA as well as from healthy volunteers. These cells were reprogrammed into induced pluripotent stem cells and then differentiated into RPE cells. The researchers were able to show that these cultured RPE cells exhibited the pigmentation defects of OCA in vitro, making them an effective model to study how lack of pigmentation affects RPE structure and function. According to the authors, these results represent a significant step toward developing novel treatments for OCA. (NIH authors: A. George, R. Sharma, T. Pfister, M. Asu-Abab, N. Hotaling, D. Bose, C. DeYoung, J. Chang, D.R. Adams, T. Cogliati, K. Bharti, and B.P. Brooks, Stem Cell Rep 17:173–186, 2022; DOI:10.1016/j.stemcr.2021.11.016)
[BY HENRY DIECKHAUS, NINDS]
NIDDK: DRUGS TARGETING SKELETAL MUSCLE METABOLISM MAY HELP TREAT DIABETES
Skeletal muscle (SKM) is responsible for more than 70% of the body’s glucose consumption, and insulin resistance in this tissue can reduce removal of sugar from the blood and lead to type 2 diabetes (T2D). In a recent study, NIDDK researchers found that clenbuterol selectively targets SKM sugar metabolism and shows promise as a potential new treatment option for people with T2D.
Clenbuterol is currently approved to treat asthma and chronic obstructive pulmonary disease and works by stimulating a receptor called the beta-2 adrenergic receptor (B2-AR), which is commonly found on the cell membranes of many types of tissue, including SKM.
In this study, the drug was fed to mice that were specially treated to induce a state of insulin resistance and elevated blood glucose, as seen in T2D. The researchers found that clenbuterol lowered blood glucose concentrations and improved whole-body glucose homeostasis despite having no effect on insulin sensitivity—a result that suggests stimulating B2-AR on the SKM membrane activates a pathway that enhances glucose metabolism. This hypothesis was supported by testing the drug protocol on genetically modified mice that lacked SKM B2-AR: Those mice did not show improved glucose tolerance with clenbuterol treatment. (NIH authors: J. Meister, D.B.J. Bone, L.F. Barella, R.J. Lee, A.H. Cohen, O. Gavrilova, Y. Cui, M. Chen, L.S. Weinstein, and Jürgen Wess, Nat Comm 13:article 22, 2022; DOI:10.1038/s41467-021-27540-w)
[BY JONATHAN CHU, NIAID]
NCI: PERSONALIZED IMMUNOTHERAPY A POTENTIAL TREATMENT FOR METASTATIC BREAST CANCER
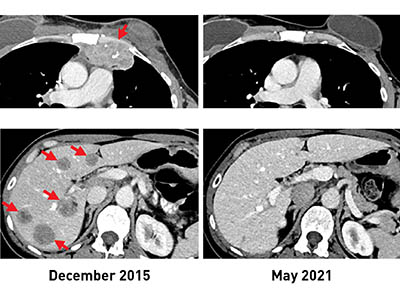
CREDIT: NCI
Before TIL therapy, a woman with breast cancer had metastatic lesions in her chest wall (top, left) and liver (bottom, left). After receiving the immunotherapy, her tumors shrank completely, and recent scans (right) show that she remains cancer free more than five years later.
Researchers at NCI have discovered a potential new avenue to treat people with hormone receptor–positive metastatic breast cancer (mBrCa), traditionally thought of as an often-incurable stage of disease with limited response to current immunotherapies.
The results of an ongoing phase 2 clinical trial now show that using a type of immune cell called tumor-infiltrating lymphocytes (TILs), which are produced by some patients, can lead to substantial mBrCa tumor regression. TILs fight cancer by recognizing a fragment of a protein on the tumor’s surface produced by specific mutations in the tumor’s DNA. The study team used whole-genome sequencing to identify these mutations in tumor samples from 42 women with mBrCa and found that 28 of the women had TIL’s that recognized their tumor.
For six of these women, the researchers grew large numbers of their mutation-specific TILs in a lab and then returned them to each patient by intravenous infusion. Three showed substantial tumor regression. These findings show the promise of personalized immunotherapy as a treatment for mBrCa and call for further studies to determine whether it can maintain a robust and durable antitumor response. (NIH authors: N. Zacharakis, L.M. Huq, S.J. Seitter, S.P. Kim, J.J. Gartner, S. Sindiri, V.K. Hill, Y.F. Li, B.C. Paria, S. Ray, B. Gasmi, C. Lee, T.D. Prickett, M.R. Parkhurst, P.F. Robbins, M.M. Langhan, T.E. Shelton, A.Y. Parikh, S.T. Levi, J.M. Hernandez, C.D. Hoang, R.M. Sherry, J.C. Yang, S.A. Feldman, S.L. Goff, and S.A. Rosenberg, J Clin Oncol 2022; DOI:10.1200/JCO.21.02170)
[BY LARISA GEARHART-SERNA, NCI]
NIDA: SUICIDES BY DRUG OVERDOSE INCREASE IN SOME GROUPS DESPITE OVERALL DECLINE
A recent NIDA-led study published in the American Journal of Psychiatry found that intentional drug-overdose deaths saw an overall decline in recent years in the United States, but increased for certain groups. The investigators discovered that suicide rates by drug overdose increased in younger men and women (ages 15–24), elderly men and women (ages 75–84), and non-Hispanic Black women of all ages.
The research team analyzed data from between 2001 and 2019 from the Centers for Disease Control and Prevention’s National Vital Statistics System and focused on data related to overdose deaths that were classified as intentional.
Moreover, the investigators found that women were more likely to die from intentional drug overdose than men, and specifically, women ages 45–64 had the highest rates of suicide by drug overdose. They also discovered that intentional drug overdose deaths occurred more in spring and summer months and were lowest during December. And more people died by intentional drug overdose on Mondays than on other days of the week.
“This research underscores the importance of external support structures and environmental factors in determining a person’s suicide risk,” said co-author Emily Einstein. (NIH authors: B. Han, W.M. Compton, E.B. Einstein, J. Cotto, J.A. Hobin, J.B. Stein, and N.D. Volkow, Am J Psychiatry 179:163–165, 2022; DOI:10.1176/appi.ajp.2021.21060604)
[BY SUNITA CHOPRA, NCI]
NHGRI, NIAMS: NEW SPECIES OF MICROBES DISCOVERED ON HUMAN SKIN
CREDIT: NHGRI
NHGRI, NIAMS: The microbiome is comprised of microorganisms that live in and on us and contribute to human health and disease. NIH researchers discovered new species of microbes that live on human skin.
The human skin is the physical barrier to foreign pathogens and plays a crucial role in maintaining an ideal microbial diversity. Shifts in this diversity are often associated with skin diseases such as acne or atopic dermatitis. NIH scientists and their colleagues recently catalogued these microbes in a massive collaborative study. The new catalog, called the Skin Microbial Genome Collection, was recently published in Nature Microbiology and successfully identifies almost 85% of the microorganisms present in a skin sample.
Newly identified were 174 bacterial species, 12 bacterial genera, and 20 jumbo phages, which are large viruses that infect bacteria. Researchers analyzed data from skin-swab samples and previously sequenced microbial samples taken from different body sites on 12 individuals. The authors used microbial culturing methods and genomic sequencing to make the new discoveries, which represent a 26% increase in the knowledge of skin bacterial diversity.
“The resource we've created will support research that explores skin health and seeks to understand the cause of these disorders,” said Julie Segre, head of the Microbial Genomics Section at NHGRI. (NIH authors: S.S. Kashaf, D.M. Proctor, C. Deming, M.E. Taylor, H.H. Kong, and J.A. Segre, Nat Microbiol 7:169–179, 2022; DOI:10.1038/s41564-021-01011-w)
[BY SATABDI NANDI, NIA]
NIDDK, NCI: BRAIN INFLUENCES INSULIN PRODUCTION

CREDIT: DIPTI AND PRAVIN KOPPIKAR, ARTSY HUES DESIGN STUDIO
This image depicts the brain as a master puppeteer manipulating several organs that regulate glucose homeostasis via neuronal projections.
A new NIH study is the first to show clear evidence of a brain-to–beta cell circuit that regulates insulin secretion in mammals. Led by NIDDK scientists, the study identified a neuronal circuit in mice that connects the brain to the beta cells of the pancreas, the cells that produce insulin. The researchers found that the circuit originates from a small set of neurons in the paraventricular nucleus of the hypothalamus, which communicate with the pancreatic beta cells to control insulin production and monitor blood glucose levels in the body.
When blood glucose became low, the neurons activated and communicated to the beta cells to stop producing insulin, preventing glucose concentrations from falling any further. Conversely, when the neurons were silenced, insulin release increased, and blood glucose dropped. The findings, which were published in Cell Metabolism, suggest that the brain elicits such protective mechanisms to overcome extreme and uncontrolled hypoglycemia. The authors propose that further research identifying similar neural circuits will advance understanding of the brain’s role in regulating blood glucose and its impact on physiology and disease. (NIH authors: I. Papazoglou, J. Lee, Z. Cui, C. Li, G. Fulgenzi, Y.J. Bahn, R.A. Piñol, M.J. Krashes, and S.G. Rane, Cell Metab 34:285–298, 2022; DOI:10.1016/j.cmet.2021.12.020)
[BY: LISA YUAN, NIDDK]
This page was last updated on Tuesday, May 17, 2022
