COVID-19 Timeline at NIH (January-February 2022)
COVID-19 Research and Activities at NIH
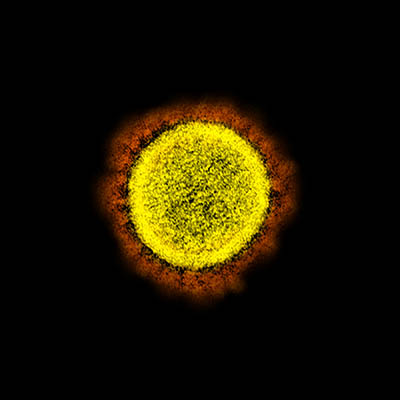
CREDIT: NIAID
Transmission electron micrograph of SARS-CoV-2 virus particles, isolated from a patient. Image captured and color-enhanced at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland.
January 1: The NIH Clinical Center implements changes to hospital operations due to significant increases in community COVID-19 cases. Urgent admissions and procedures will be prioritized, and procedures of a less urgent nature will be deferred or rescheduled to a later time.
January 3: NIH adopts the updated CDC guidance issued on December 27 for shortened isolation and quarantine periods for employees testing positive for or exposed to COVID-19. People with COVID-19 should isolate for five days and if they are asymptomatic or their symptoms are resolving (without fever for 24 hours), wear a mask when around others for an additional five days. Safety measures at NIH now require the use of a special type of face mask, called an ASTM Level 3 mask for those working on-site.
January 4: All non-mission-critical travel is restricted again. Travel initiated after January 12, 2022, must be approved by the NIH Deputy Director for Management.
January 4: The “NIH Director’s Blog” features Science’s biomedical breakthroughs of 2021 that NIH played some role in advancing. These include artificial antibody therapies and antiviral pills to treat COVID-19, and artificial intelligence approaches that helped to predict structural changes in spike proteins of SARS-CoV-2 variants delta and omicron.
January 6: An NIH-funded study found that women receiving one dose of a COVID-19 vaccine during a single menstrual cycle had an increase in cycle length of nearly one day compared with unvaccinated women. (Obstet Gynecol Jan 5, 2022)
January 7: NIH’s Division of Occupational Health and Safety clarifies mask guidance issued on January 3 for staff returning to work onsite: ASTM Level 3 masks are only mandated for staff in health care settings, staff returning after a positive COVID test or exposure, and everyone entering the Clinical Center, where masks will be provided upon entry into the hospital. Staff who work in certain patient care and laboratory functions with potential high-risk COVID-19 exposures are already enrolled in the NIH Respiratory Protection Program and are issued and required to use N-95 or higher-level respirators during high-risk activities. Employees working in administrative functions other than a lab setting can choose a well-fitting mask, including cloth masks. More detailed information can be found on the NIH Safety Guidance & COVID-19 Safety Plan at https://go.usa.gov/xtYrW.
January 7: NIH Acting Director Lawrence Tabak emails staff to announce that the ninth virtual town hall will be held on January 27 to update staff on the state of the pandemic and return-to-the-physical-workspace plans. He summarizes the operational and guidance changes at NIH issued earlier this week and reports that NIH COVID-19 testing services will now be reserved only for staff who are required to or have been approved to work on-site.
January 14: The CDC updates its mask guidance to clarify that surgical masks and respirators such as N-95s and KN-95s offer better protection from SARS CoV-2 compared with cloth masks.
January 18: To comply with the Executive Order issued on September 9, 2021, all non-patient visitors arriving at an NIH facility will be required to attest to their vaccination status. Visitors who are not fully vaccinated must provide proof of a negative COVID-19 test result within the past 72 hours to gain access to NIH property. Enforcement will begin on Monday, January 24, to allow for a period of transition.
January 18: A study led by NICHD investigators finds that SARS-CoV-2 infection during pregnancy may cause inflammatory immune responses in the fetus, even if the virus does not infect the placenta. (Nat Comm 13:article 320, 2022)
January 18: On the “NIH Director’s Blog,” Acting NIH Director Lawrence Tabak, who also runs a lab at the National Institute of Dental and Craniofacial Research (NIDCR), reports on a collaboration with Kelly Ten Hagen (NIDCR) that demonstrated how O-glycosylation can influence SARS-CoV-2, the coronavirus that causes COVID-19, and its ability to fuse to cells, which is a key step in infecting them. The alpha and delta variants carry a spike mutation that could mean decreased O-glycosylation (Proc Natl Acad Sci U S A 118:e2109905118, 2021). Tabak notes that the omicron variant (which was not studied in the cited paper) carries the very same spike mutation as well as another change that might further alter O-glycosylation. The Ten Hagen lab is looking into these leads to learn how this region in omicron affects spike glycosylation and, ultimately, the ability of SARS-CoV-2 to infect human cells and spread.
January 20: COVID-19 vaccination does not affect the chances of conceiving a child, according to an NIH-funded study of more than 2,000 couples. However, couples had a slightly lower chance of conception if the male partner had been infected with SARS-CoV-2 within 60 days before a menstrual cycle, suggesting that COVID-19 could temporarily reduce male fertility. (Am J Epidemiol kwac011, 2022)
January 20: In a new viewpoint essay published today in Science, Avindra Nath, clinical director of NINDS, and Serena Spudich from the Yale School of Medicine (New Haven, Connecticut) highlight what is currently known about the effects of SARS-CoV-2 on the brain, the importance of increased research into the underlying causes of long COVID, and possible ways to treat its symptoms. (Science 375:267–269, 2022)
January 21: NIH implements new COVID-19 screening guidance and encourages all contractors with a PIV card to upload their proof of COVID-19 vaccination by February 15, 2022, to ensure ongoing access to NIH facilities. Contractors who elect not to upload proof of vaccination by February 15 will be required to enter through an NIH visitor entrance where they will be required to verbally attest to their primary vaccination status. Contractors who are unvaccinated, partially vaccinated, or choose not to report their vaccination status will be required (every time they enter an NIH property or facility) to provide a physical or digital negative COVID-19 test result (at-home tests will not be accepted) within the past 72 hours to gain access to the facility.
January 21: The NIH Office of Intramural Training and Education urges all trainees who are appointed using contract mechanisms to upload their proof of COVID-19 vaccination by January 31, 2022. Trainees appointed after January 31, 2022, must provide proof that they have been vaccinated against COVID-19 prior to their first day of active work at the NIH unless they have an approved exception. Visiting fellows who are unable to obtain an appropriate vaccine in their home country may be on-boarded and work remotely in the United States for up to 60 days until they comply with the NIH vaccine requirement.
January 21: NIH Acting Director Lawrence Tabak emails all staff with optimism that the omicron surge is on a downward trend nationally, based on data from the CDC. He highlights recent NIH-supported research on reproductive health as well as a study that found that boosters are needed to protect against the omicron variant. He mentions the national court decision issued today places a temporary hold on the implementation and enforcement of the federal employee vaccine mandate. The Department of Justice has appealed this decision, and NIH is awaiting further guidance. Tabak reviews the vaccination reporting requirements for on-site contractors issued today, as well as the CDC guidance on isolation and quarantine periods adopted by NIH on January 3. NIH again updates its mask guidance issued on January 7: It’s recommended that all staff working on-site at an NIH facility now wear well-fitting, disposable surgical masks, which are readily available through the NIH Supply Center. Cloth masks alone are not allowed at this time.
January 24: An NIH-funded study finds that social connectedness, sleep, and physical activity were associated with better mental health among youth during the COVID-19 pandemic. (J Adolesc Health 70:387–395, 2022)
January 26: A clinical trial sponsored by NIH finds that in adults who had previously received a full regimen of any of the three COVID-19 vaccines currently authorized by the FDA, an additional booster dose of any of these vaccines was safe and prompted an immune response. (New Engl J Med 2022; DOI:10.1056/NEJMoa2116414)
January 26: A cloud-based computer analysis of existing genomic data uploaded by NIH to a global sequence database uncovers 100,000 novel viruses, including nine new types of coronaviruses similar to SARS-CoV-2. The database contains 16 petabytes of archived sequences, which come from genetic surveys of everything from fugu fish to farm soils to the insides of human guts. (Nature 602:142–147, 2022)
January 27: Acting NIH Director Lawrence Tabak hosts the ninth virtual Town Hall with Acting Principal Deputy Director Tara Schwetz, NIAID Director Anthony Fauci, Deputy Director for Management Alfred Johnson, and NIH Chief People Officer Julie Berko, with over 10,000 attending online. The NIH leaders answer questions and provide an update on the Office of the Director leadership transition, the current state of the pandemic, and changes to travel, services, and return-to-the-physical-workplace plans due to surges in COVID-19 cases. Videocast (HHS only) at https://videocast.nih.gov/watch=44500
January 27: The guidance issued on January 21 related to NIH contractor access to NIH facilities and properties based on vaccination status is paused as a result of the preliminary nationwide court injunction.
January 27: An NIAID-supported clinical trial finds that the combination of remdesivir plus a highly concentrated solution of antibodies that neutralize SARS-CoV-2 is not more effective than remdesivir alone for treating adults hospitalized with COVID-19. (Lancet 399:530–540, 2022)
January 28: HHS Secretary Xavier Becerra emails all staff announcing a new video from the We Can Do This Campaign about the importance of getting boosted.
January 31: NIH permits non-mission-critical travel to resume with approval from the traveler’s institute or center.
January 31: NIH announces that an NIAID-supported study will assess whether temporarily reducing immunosuppressive medication taken during the days before and after an additional dose of an mRNA COVID-19 vaccine safely allows for better antibody response to vaccination in kidney- and liver-transplant recipients.
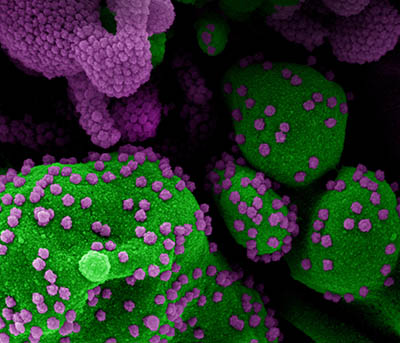
CREDIT: NIAID
Colorized scanning electron micrograph of an apoptotic cell (green) heavily infected with SARS-CoV-2 virus particles (purple), isolated from a patient sample. Image from the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland.
February 1: The “NIH Director’s Blog” features a promising study demonstrating how a specially engineered protein particle can neutralize multiple SARS-CoV-2 variants in a mouse model. The protein mimics the 3D structure of the receptor for angiotensin converting enzyme, a protein on the surface of our cells that the virus’s spike proteins bind to as the first step in causing an infection. (Nat Chem Biol 2022; DOI:10.1038/s41589-021-00965-6)
February 2: NIAID announces a new pandemic preparedness plan targeting known viruses and identifying viral threats before they emerge: https://www.niaid.nih.gov/research/pandemic-preparedness
February 3: NIH begins the voluntary At-Home Antigen Testing Pilot Program as part of continued efforts to mitigate workplace transmission of COVID-19. Eligible staff who are reporting on-site at an NIH facility may receive at least an eight-week supply of rapid COVID-19 test kits at no cost. Tests may be ordered by completing the online test kit order form (https://safercovid.org/mytest/nihorder.html) or picked up at NIH property locations.
February 4: In his weekly email to staff, NIH Acting Director Lawrence Tabak reiterates mask guidance updated on January 21 as well as yesterday’s announcement of the NIH at-home COVID-19 testing program. He highlights this week’s new research from NIAID and their new pandemic preparedness plan.
February 7: An NICHD-funded study finds that pregnant women with moderate to severe COVID-19 infection appear to be at greater risk for common pregnancy complications than uninfected women. Mild or asymptomatic infection was not associated with increased pregnancy risks. (JAMA 327:748–759, 2022)
February 9: HHS Deputy Secretary Andrea Palm emails staff to announce the next phase of the return-to-the-workplace process. Phase 2B employees will return beginning March 27, 2022, and Phase 2C employees will return beginning April 10, 2022.
February 9: In an email to all staff, NIH Acting Director Lawrence Tabak reviews today’s HHS announcement that the return to on-site work process will resume. NIH federal employees in Phase 2B will receive official notices on February 10 that their return to on-site work will begin on March 27. Phase 2C staff, which includes all remaining NIH federal employees, will receive notices on February 24 that their on-site work is anticipated to begin on April 10. Tabak expects the process will be gradual and says that NIH leadership is exploring the use of continued workplace flexibilities. He also anticipates the issuance of a new HHS Workplace Flexibilities Policy in the coming weeks that will provide guidance on telework, remote work, and flexible schedules.
February 11: NIH resumes normal COVID-19 reporting procedures for positive COVID-19 test results, which had been temporarily suspended for people working off-site during the omicron surge. All staff, including teleworkers and remote workers, are encouraged to report a new diagnosis of COVID-19 infection, including diagnoses received in the community or from at-home tests using the online Coronavirus Surveillance Questionnaire at https://ors.od.nih.gov/Pages/oms-covid-screening.aspx.
February 14: NIH increases the allowable density for primary facilities around the country: Maryland locations will be allowed one person per 35 square feet (in July 2020, it was 1 person per 125 square feet). Density now allowed in other NIH locations: Phoenix, Arizona, 1 person per 75 square feet; Hamilton, Montana, 1 person per 50 square feet; and North Carolina locations, 1 person per 75 square feet. Density requirements will be reassessed the first Tuesday of each month based on local COVID-19 case rates and conditions.
February 15: NIH launches a COVID-19 facts campaign on social media under the tag #NIHCOVIDFacts to educate and dispel common COVID-19 myths and highlight NIH’s role in the pandemic.
February 16: President Joseph Biden announces that former NIH Director Francis Collins will temporarily perform the duties of science advisor to the President and co-chair of the President’s Council of Advisors on Science and Technology until permanent leadership is nominated and confirmed.
February 16: John Mascola announces his retirement as director of the Vaccine Research Center (VRC), effective at the end of March. Mascola and his team have been instrumental in combating some of the greatest public health threats of our time, including HIV, Ebola, and malaria. Most notably, he and his team at the VRC collaborated with Moderna to develop the mRNA-1273 COVID-19 vaccine that has now been administered to millions of people in the United States. Richard Koup, the current deputy director of the VRC, will serve as acting VRC director.
February 17: An NHLBI-led study identifies types of autoantibodies, which target a person’s own organs and systems, that correlate with severe COVID-19 illness and may help explain mechanisms associated with severe blood clotting. (Arthritis Rheumatol 2022; DOI:https://doi.org/10.1002/art.42094)
February 18: NIH Acting Director Lawrence Tabak emails all staff to remind them that NIH mask requirements remain in place, even as county and state mask mandates are lifted. He reviews this week’s announcement regarding the increase in allowable density for NIH facilities, as well as last week’s HHS announcement for returning to the physical workplace and resuming normal reporting procedures for positive COVID-19 test results. Tabak encourages staff to take advantage of the Employee Support Line and announces that the NIH Employee Assistance Program is hosting a special three-part webinar series titled “Emotional Preparation for Returning to the Physical Workplace,” which will address barriers and worries associated with the return. The first session is Thursday, February 24.
February 22: The NIH Clinical Center resumes walk-in appointments for asymptomatic COVID-19 testing services for staff working on-site at an NIH facility, which had been paused during the omicron surge.
February 23: HHS Assistant Secretary for Administration Cheryl Campbell emails staff to announce a new COVID-19 screening testing program for employees who are not fully vaccinated and must report onsite, interact in person with members of the public, or be tested as part of their job duties. Employees who have not logged their vaccination status in their division’s collection system will also be subject to screening testing, and specific testing protocols will be communicated by each division’s leadership.
February 24: A study led by NIMHD finds that people from all major racial and ethnic minority population groups in the United States report experiencing more COVID-19–related discrimination than white adults. People from groups that have been marginalized, such as those who speak little to no English and those with lower levels of education, were also found to face more discrimination due to the pandemic. (Am J Public Health 112:453–466, 2022; DOI:10.2105/AJPH.2021.306594)
This page was last updated on Tuesday, May 17, 2022
