Research Briefs
NIAID, NCI, NCATS, NIEHS: EXPERIMENTAL TREATMENT FOR CHILDREN WITH ECZEMA
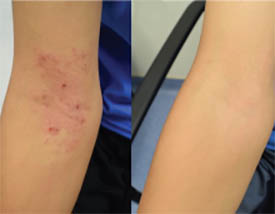
CREDIT: NIAID
Inner elbow of a child with eczema before Roseomonas mucosa therapy (left) and after four months of treatment (right).
Atopic dermatitis, also called eczema, is a chronic inflammatory skin disease characterized by dry, itchy skin and rashes. NIH researchers found that an experimental treatment that modifies the skin microbiome safely reduced disease severity and increased quality of life for children as young as three years old. Eczema, which is most common in children, affects about 5% to 25% of children worldwide and is linked to an increased risk of developing asthma, hay fever, and food allergy.
Eczema is associated with an imbalance of the resident skin microbiome. Previous studies have found topical application of Roseomonas mucosa, a bacterium found naturally on healthy skin, is beneficial, but haven’t yielded insights into the molecular mechanisms of how R. mucosa improves clinical symptoms or its therapeutic effects in children younger than 7 years old (the most common age group for eczema patients).
In an open-label clinical trial with children aged 3 years or older, NIH researchers found R. mucosa treatment is associated with clinical improvement of eczema-related symptoms and the skin’s barrier function; a decline in itching and use of topical steroids; increased microbial diversity; and decreased amounts of Staphylococcus aureus, a bacterium known to exacerbate eczema. Most important, continued clinical improvement was noticed up to eight months after the treatment was discontinued.
Digging deep to find the molecular mechanisms, NIH researchers used ribosomal RNA sequencing, which confirmed the shift toward a healthy skin microbiome population after the treatment. They also found that a specific set of lipids produced by R. mucosa strains isolated from healthy skin can induce skin-repair processes and promote the turnover of skin tissue. Overall the study provides a fundamental basis to initiate a larger randomized and placebo-controlled clinical trial. (NIH authors: I.A. Myles, C.R. Castillo, K.D. Barbian, K. Kanakabandi, K. Virtaneva, E. Fitzmeyer, M. Paneru, F. Otaizo-Carrasquero, T.G. Myers, T.E. Markowitz, I.N. Moore, X. Liu, M. Ferrer, Y. Sakamachi, S. Garantziotis, M. Swamydas, M.S. Lionakis, E.D. Anderson, N.J. Earland, S. Ganesan, A.A. Sun, J.R.E. Bergerson, C.A. Martens, and S.K. Datta, Sci Transl Med 12(issue 560):eaaz8631, 2020; DOI:10.1126/scitranslmed.aaz8631)
NICHD: OPIOID USE MAY BE LINKED TO PREGNANCY LOSS, LOWER CHANCE OF CONCEPTION
Though physicians often prescribe opioids to women of reproductive age, including after a cesarean or vaginal delivery or following a miscarriage, little is known about the effects of the periodic use of such drugs in women who were previously pregnant and wish to conceive again. Researchers in NICHD aimed to answer this question by examining the trends of conception and miscarriage in 1,228 women who had had one or two previous pregnancy losses and were trying to get pregnant again. The research team monitored prescription opioid use in these women through urine samples and self-reporting. Of these participants, 226 (18%) used opioids while trying to conceive; among 683 women who had pregnancies lasting at least eight weeks, 33 (5%) had positive urine detection of opioids during early pregnancy. For women who used opioids while trying to conceive, the researchers found a 29% reduced likelihood of achieving conception. Among the women who became pregnant, those who used opioids around the time of conception were 1.5 times as likely to have a miscarriage as women who had not. Further, those who used opioids during the first four weeks of pregnancy were more than twice as likely to have a miscarriage, with risk increasing the later into pregnancy opioids were used.
The results highlight the need for patients and physicians to carefully weigh the benefits and drawbacks of opioid use while trying to conceive, but more research is needed before any specific recommendations can be made. (NICHD authors: K.S. Flannagan, S.L. Mumford, L.A. Sjaarda, J.G. Radoc, N.J. Perkins, V.C. Andriessen, J.R. Zolton, and E.F. Schisterman, Epidemiology 31:844–851, 2020; DOI:10.1097/EDE.0000000000001247)
NHLBI: STUDY SHOWS DECLINE IN AWARENESS, TREATMENT, AND CONTROL OF HIGH BLOOD PRESSURE
In the past 15 years, there’s been a concerning downward trend in awareness among Americans about hypertension (high blood pressure) and how to control and treat it, according to an NHLBI-funded study that included one intramural investigator. Hypertension can be controlled by making lifestyle changes—such as losing weight, exercising regularly, eating a healthy diet—and by taking antihypertensive medications. Proper control of blood pressure reduces the risk of cardiovascular disease and stroke.
The study analyzed data from the National Health and Nutritional Examination Survey of more than 18,000 American adults 18 and older with hypertension, which was defined as 140/90 millimeters (mm) of mercury (Hg) or higher at the time of the study; now the definition is 130/80 mm Hg or higher. The researchers found 70% of participants were aware of their condition in 1999–2000; awareness increased steadily to 85% in 2013–2014 but declined to 77% in 2017–2018. Of all adults with high blood pressure, the number who managed to control their condition increased from 32% in 1999–2000 to 54% in 2013–2014, but then declined to 44% in 2017–2018. Of those, the number taking blood-pressure medications increased from 53% in 1999–2000 to 72% in 2013–2014, then declined to 65% in 2017–2018. The study also found that a significantly lower proportion of non-Hispanic Black adults had controlled blood pressure versus non-Hispanic white adults.
It’s important to develop “more effective strategies to reverse and substantially improve blood pressure control,” said study co-author Lawrence Fine (NHLBI) in a press release. (NHLBI author: L.J. Fine, JAMA 324:1190–1200, 2020; DOI:10.1001/jama.2020.14545)
NCCIH, NIMH, NINR, NINDS: POST-EXERTIONAL MALAISE IN CHRONIC FATIGUE SYNDROME
Myalic encephalitis/chronic fatigue syndrome (ME/CFS) is a debilitating, chronic disease characterized by pain, cognitive difficulties, and severe fatigue. Scientists cannot fully explain this disease, which may affect between 836,000 and 2.5 million people in the United States. One of its major symptoms is post-exertional malaise (PEM), the worsening of symptoms after physical or mental activities. To get a better understanding of the syndrome, NIH researchers conducted a study in which 43 ME/CFS patients described their experiences.
The patients were divided into nine focus groups and discussed their daily activities that led up to PEM, its duration, and the techniques they used to minimize symptoms. Five out of the nine focus groups included people who experienced PEM after a cardiopulmonary exercise test (CPET). Many of the PEM symptoms fell into three major categories: exhaustion, neuromuscular trouble, and cognitive difficulties. Other symptoms included nausea, headaches, pain, sore throat, and sensitivity to light and sound. PEM symptoms occurring in patients’ day-to-day lives appeared within 24–48 hours after an activity and lasted from 24 hours to several weeks. In patients who had taken part in the CPET activity, the onset of PEM after the test was faster, lasted longer, and was more severe. Nearly all the patients said they needed complete rest in a quiet, dark room to minimize the symptoms. The researchers concluded that the PEM symptoms varied widely within individual patients. The study also identified, for the first time, differences between PEM caused by daily activities and by the CPET test. More research is needed to identify PEM subtypes, which may help guide the development of targeted treatments. (NIH authors: B. Stussman, A. Williams, J. Snow, A. Gavin, R. Scott, A. Nath, and B. Walitt, Front Neurol 11:article 1025, 2020; DOI:10.3389/fneur.2020.01025)
CC, NICHD: MIGLUSTAT MAY DECREASE RISK OF PNEUMONIA AND DEATH IN CHILDREN AND ADOLESCENTS WITH NIEMANN-PICK DISEASE TYPE C1
NIH researchers found that a drug called miglustat seemed to stabilize swallowing problems in children and adolescents with Niemann-Pick disease type C1 (NPC1) and decrease the risk of pneumonia caused by the aspiration of food or drink. NPC1, which has no FDA-approved therapy, is a rare genetic disorder that causes a progressive decline in neurological and cognitive functions. Although previous studies suggested that the off-label use of miglustat helps stabilize swallowing function in NPC1 patients, they did not document swallowing safety with one’s ability to eat and drink or with aspiration risk.
In the current observational study, the researchers used video fluoroscopy—a digitized X-ray movie—to examine the ability of miglustat to improve the swallowing function in 50 children and adolescents with NPC1. Scans were ranked based on the likelihood and extent of food and drink entering the airway. On average, each patient was evaluated once a year for three years. By the end of the study, 36 patients had been prescribed miglustat and 24 had not. A longitudinal analysis found that, compared with those not taking miglustat, those who took the drug had a 91% lower risk for deterioration of swallowing and a 72% lower risk of aspiration. Because the FDA has not approved the use of miglustat for the treatment of NPC1, many insurers hesitate to cover its cost, thus reducing its availability to patients in the United States. The findings from this study add to growing clinical evidence demonstrating the efficacy of miglustat for treating NPC1 and support its use in this population. (NIH authors: B.I. Solomon, A.C. Smith, A.C., N. Sinaii, N. Farhat, M.C. King, L. Machielse, and F.D. Porter, JAMA Neurol, 2020; DOI:10.1001/jamaneurol.2020.3241)
NIA: MACHINE LEARNING DETECTS EARLY SIGNS OF OSTEOARTHRITIS
Osteoarthritis is a type of arthritis resulting from worn down cartilage in the joints. Although osteoarthritis is the most common form of arthritis, it’s not usually diagnosed until after the damage is done. NIA researchers, in collaboration with scientists from other institutions, found that artificial intelligence (AI) and magnetic resonance imaging (MRI) scans could help predict signs of osteoarthritis three years before diagnosis.
The researchers analyzed MRI scans of 86 people, who had no symptoms of osteoarthritis; half, however, developed the condition after three years. The scientists developed a technique called three-dimensional transport-based morphometry (3D TBM) that used MRI scans to show biochemical chances, such as the presence of water, in knee cartilage. The researchers used machine-learning algorithms to train the AI system to distinguish between the cartilage of those who would and wouldn’t develop osteoarthritis.
This system ended up accurately detecting 78% of pre-symptomatic cases. More studies need to be done, however, to determine if this system would be useful for diagnostics and identify people who would be helped by early interventions. (NIA authors: B.G. Ashinsky, M. Bouhrara, and R.G. Spencer, Proc Natl Acad Sci U S A, 117:24709-24719, 2020; DOI:10.1073/pnas.1917405117)
This page was last updated on Monday, March 21, 2022
