Remdesivir Is Active Against MERS Coronavirus in Monkeys
Drug Also Being Tested in Clinical Trials Against the New Coronavirus
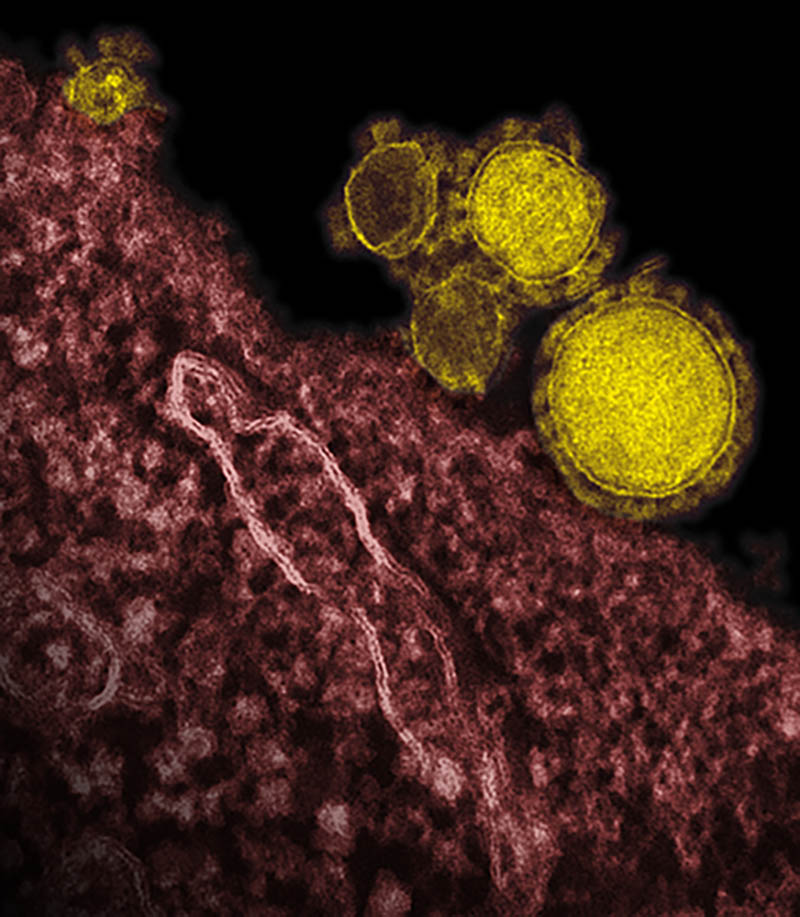
CREDIT: ROCKY MOUNTAIN LABS, NIAID
This electron micrograph shows Middle East respiratory syndrome coronavirus particles budding from a cell. The spikes around the surface of the particles give coronaviruses their name, crown-like.
The drug remdesivir has antiviral effects against a variety of viruses including Middle East respiratory syndrome coronavirus (MERS-CoV) in rhesus monkeys (Macaca mulatta), according to a study conducted by NIAID researchers at the Rocky Mountain Labs in Hamilton, Montana.
“The finding is very significant because people diagnosed with MERS-CoV develop severe respiratory disease, and one out of three do not survive. Therefore, an effective treatment could make a difference in disease outcome in MERS patients if treated early after diagnosis,” said Emmie de Wit, first author on the study, which appeared in Proceedings of the National Academy of Sciences U.S.A. “Because MERS-CoV and [the new COVID-19] are closely related viruses, we have reason to think remdesivir treatment will work against the new coronavirus as well.”
In the study, one group of animals treated with the drug 24 hours before the virus was introduced developed no clinical signs of infection. A second group, treated 12 hours after being infected, showed less severe disease than the control group.
“I was worried that a treatment given after infection would not have enough time to limit virus replication,” said de Wit. “But we still saw a positive effect of therapeutic remdesivir treatment…and a reduction in damage to the lungs.”
The World Health Organization has classified MERS-CoV, which was first discovered in Saudi Arabia in 2012, as a disease with the potential to cause epidemics. Therefore, there is an urgent need for the development of effective antivirals for its treatment and prevention. Remdesivir has previously shown efficacy in the treatment of Ebola virus in monkeys and the severe acute respiratory syndrome coronavirus (SARS-CoV) in mice. The scientists indicate that the promising study results support additional clinical trials of remdesivir for MERS-CoV and COVID-19. At least two clinical trials of remdesivir for COVID-19 are underway in China, and other patients with COVID-19 infection have received the drug under a compassionate-use protocol.
(NIH authors: E. de Wit, F. Feldmann, J. Cronin, T. Thomas, D. Scott, and H. Feldmann, Proc Natl Acad Sci USA, 2019; DOI:10.1073/pnas.1922083117)
This page was last updated on Tuesday, March 29, 2022
