Creating Devices for the Clinic
Deciding Which Seeds Are Worth Watering
For every great idea–for every great solution to a problem–there are a thousand ideas that fall by the wayside, discarded along the path that begins at the first flash of insight and ends in a working solution. Translational medicine, in which an idea for a clinical solution is shifted from the laboratory testing ground to the public, often follows this twisty path, especially in the realm of developing new medical devices.
The interdisciplinary NIH Center for Interventional Oncology—a collaboration among the NIH Clinical Center, the National Cancer Institute, and the National Institute of Biomedical Imaging and Bioengineering—was created in 2009 to foster just such translational work. The center develops targeted-imaging technologies to diagnose and/or treat various forms of cancer.
“Our process is definitely one that’s hard to grasp,” said center director Bradford Wood. The interdisciplinary approach favored in the center depends in part on developing or matching up tools that may have been intended for other purposes, and putting them together in novel ways to address an unmet clinical need. It’s important to have “the resources, people and expertise on our team that might be able to build a match for that need,” Wood added.
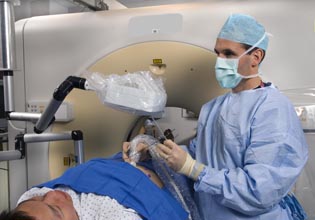
PHOTO CREDIT: CENTER FOR INTERVENTIONAL RADIOLOGY
Bradford Wood is using a fusion-imaging technique developed by NIH’s Center for Interventional Oncology. The technique involves superimposing a magnetic-resonance-imaging scan on a real-time ultrasound image to create a detailed map of an internal area to be biopsied or treated. As Wood looks at a computer screen showing the map, he can guide the needle to the appropriate areas.
As a strategy to filter the most promising leads, Wood’s group takes on many projects simultaneously, nurturing a variety of ideas to see which ones take off. “Deciding which of those seeds is worth watering and worth following through is both the hard part and the fun part of what we do,” said Wood. “And those decisions are made as a group or by the technology itself.”
That means that at any one time, the group is balancing projects at every stage, from fledgling ideas, to proof-of-concept prototypes, to devices and techniques that are in clinical trials or in the process of commercialization or translation to the community.
One of their best-known projects—now commercialized after clinical trials—is a new technique for image-guided prostate biopsies whereby physicians fuse two modes of imaging to locate possible cancerous tissue. Previously, in cases of suspected prostate cancer, biopsies were taken randomly and blindly throughout the prostate. Wood’s group developed new software that superimposes a magnetic-resonance-imaging scan on a real-time ultrasound image to create a detailed multi-modality map of the prostate. The clinicians (Wood and Peter Pinto) can then use the map to guide the biopsy needle to suspicious-looking areas (identified by NCI Molecular Imaging Program partners Peter Choyke and Baris Turkbey) that are likely to be cancerous lesions. And although the exact clinical indications and roles are evolving, “the evidence seems to be pretty clear that this is an improved way to biopsy the prostate,” said Clinical Center senior investigator Ronald Summers, who’s working on automated ways of using MRI to diagnose or facilitate the diagnosis of prostate cancer.
Another of the center’s long-term projects involves using liposome nanoparticles as a chemotherapy drug-delivery system and applying heat to get them to release the drugs in the area of a tumor. Thermal-sensitive liposomes have been around for many years. Researchers would apply hot packs or other external heat sources over an affected area; when the liposomes traveled via the bloodstream to the heated area, they would melt and release their contents.
Wood’s group, however, developed a more precise method: They paired the drug-bearing liposomes with radiofrequency ablation (RFA) or high- intensity-focused ultrasound (HIFU), which produces a small amount of heat in a narrowly defined space. So the drug is released at a high concentration exactly where it’s needed most. The group has used the HIFU technique to deliver chemotherapy drugs directly to cancer sites in rabbit models, and RFA in patients (now in phase 3 international clinical trials). “We’re still trying to fully understand the implications of HIFU hyperthermia,” said Wood. “It improves oxygenation, which can cause more free radicals and can increase sensitivity to radiation. It also increases blood flow, so mechanical, thermal, and chemical synergy all increase drug delivery!”
The team has also pioneered other applications for image-guided drug delivery, including the embolization of beads that are both drug-eluting and imageable and are about 100 microns in size. The technology was co-developed with a CRADA (cooperative research and development agreement) public-private partnership and is being commercialized for release soon. These imageable drugs can be localized and tracked with navigation “video-game-like” X-ray software that makes use of fusion technology, similar to the prostate project, to compare drug dose to anatomy to tumor location.
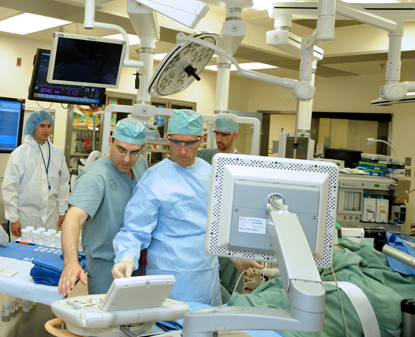
PHOTO CREDIT: CENTER FOR INTERVENTIONAL RADIOLOGY
Close collaborations enable new perspectives on procedural medicine. In foreground: Peter Pinto (left) and Bradford Wood.
The center’s project mix includes new methods of combating bacterial contamination on catheters and other medical devices. “We had a bit of a crazy idea to design an electrified catheter with super weak electric current to inhibit bacterial colonization,” said Wood. Some bacteria form antibiotic-resistant biofilms that can colonize the catheters and cause life-threatening bloodstream infections. Drawing on knowledge from a variety of disciplines, Wood’s group designed a device with a very low electrical current–low enough not to cause heart arrhythmias–and showed that a continuous current across the tip of the device could significantly reduce the formation of bacterial biofilms.
The Center for Interventional Oncology thrives on the interdisciplinary environment at the Clinical Center and draws on expertise from many academic and industry partners to develop its technologies. “Every day I learn something from a great enlightening conversation with somebody in a different discipline,” said Wood. “You think, that’s a cool unique way to think about the problem!”
PHOTO CREDIT: CENTER FOR INTERVENTIONAL RADIOLOGY
The Center for Interventional Oncology, led by Bradford Wood, has pioneered a number of medical techniques including applications for image-guided drug delivery; pairing drug-bearing liposomes with radiofrequency ablation or high-intensity-focused ultrasound to make them more effective; and designing catheters to emit a low-level electrical current that would inhibit bacterial contamination.
“There are a lot of different paradigms for successful contributions in cancer diagnosis and treatment,” said Summers. For the Center for Interventional Oncology, interdisciplinary team science “has been a successful approach.”
Not every crazy idea ends up in patients or as a successful commercialized product. Wood’s lab is full of failed contraptions that sit in the “device graveyard” without a clinical home. “We have a lot of projects that are high-risk and high-yield, in various phases of feasibility or proof of concept,” said Wood. “Most of those will die a timely death.” For example, there are multiple CT-integrated robots and carpentry-like devices in his closets that fit this description and were never judged to be clinically valuable or cost-effective.
But some of these “crazy idea” seeds will be watered and eventually blossom into working clinical solutions.
To watch a videocast of Bradford Wood’s May 13, 2015, presentation, “Video Game Medicine at the NIH: How Image-Guided Therapies Depend upon Interdisciplinary Translational Team Sc*ience,” at a Clinical Center Grand Rounds, go to http://videocast.nih.gov/launch.asp?19000.
More about the Center for Interventional Oncology
NIH Record (July 17, 2015): https://nihrecord.nih.gov/sites/recordNIH/files/pdf/2015/NIH-Record-2015-07-17.pdf
NIH press release (April 22, 2009): http://www.nih.gov/news/health/apr2009/cc-22.htm
NIH Catalyst (March-April 2009): https://nihsearch.cit.nih.gov/catalyst/2010/10.04.01/catalyst_v18i2.pdf
REFERENCES
Fusion-guided prostate biopsyFusion-guided prostate biopsy
M.M. Siddiqui, S. Rais-Bahrami, B. Turkbey, A.K. George, J. Rothwax, N. Shakir, et al., “Comparison of MR/ultrasound fusion–guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer,” JAMA 313: 390–397 (2015).
"Medical GPS" smart needles & fusion ablation
J. Krücker, S. Xu, A. Venkatesan, J. Locklin, H. Amalou, N. Glossop, B.J. Wood, “Clinical Utility of Real-Time Fusion Guidance for Biopsy and Ablation,” J Vasc Interv Radiol 22:515–524 (2011)
Heat-sensitive liposomes with doxorubicin activated by RFA (Phase I trial)
B.J.Wood, R.T. Poon, J. Locklin, M.R. Dreher, K.K. Ng, M. Eugeni, G. Seidel, S. Dromi, Z. Neeman, M. Kolf, C.D.V. Black, R. Prabhakar, S.K. Libutti, “A Phase I Study of Heat Deployed Liposomal Doxorubicin during Radiofrequency Ablation for Hepatic Malignancies,” J Vasc Interv Radiol. 2:248–255 (2012)
Heat-sensitive liposomes with doxorubicin activated by HIFU
S. Dromi, V. Frenkel, A. Luk, B. Traughber, M. Angstadt, M. Bur, J. Poff J, Xie, S.K. Libutti, K.C.P. Li, B.J. Wood, “Pulsed-high intensity focused ultrasound and low temperature sensitive liposomes for enhanced targeted drug delivery and anti-tumor effect,” Clin Cancer Res 13:2722–2727 (2007)
Image-able drug-eluting microbeads for liver cancer
M.R. Dreher, K.V. Sharma, D.L. Woods, G. Reddy, Y. Tang, W.F. Pritchard, O.A. Chiesa, J.W. Karanian, J.A. Esparza, D. Donahue, E.B. Levy, S.L. Willis, A.L. Lewis, B.J. Wood, “Radiopaque Drug-Eluting Beads for Transcatheter Embolotherapy: Experimental Study of Drug Penetration and Coverage in Swine,” J Vasc Interv Radiol 2:257–264 (2012)
Electrified catheter to inhibit catheter related bloodstream infections
H. Amalou, A.H. Negussie, A. Ranjan, L. Chow, S. Xu, C. Kroeger, et al., “Electrically conductive catheter inhibits bacterial colonization,” J Vasc Interv Radiol 25: 797–802 (2014)
This page was last updated on Monday, April 25, 2022
