Research Briefs
NIDCR: REGENERATING TEETH
NIDCR researchers were part of an NIH-Harvard team that was the first to demonstrate the ability to use a low-power laser light (LPL) to coax stem cells inside the body to regenerate tissue. They used a small dose of LPL to activate dental stem cells in rat molars to generate dentin, the bonelike tissue that is major component of teeth. The rats had cavities in two molars—both cavities were filled, but one was treated with LPL and the other wasn’t. The researchers found that dentin was induced in the LPL-treated molar after 12 weeks. The researchers also outlined the molecular mechanism involved: They found that LPL treatment generated a type of molecule known as reactive oxygen species, which stimulated dentin production by activating transforming growth factor–beta, a signaling protein that can promote dental stem-cell differentiation. The researchers also showed that LPL induced adult human dental stem cells to form dentin in the laboratory. The findings may lead to new approaches to develop low-cost, noninvasive therapies for treating dental disease and tooth damage. The lead author, who was a postdoc at Harvard at the time of the study, is now at NIDCR. (NIDCR authors: P.R. Arany, A. Cho, and A. Kulkarni, Sci Trans Med 6:238ra69, 2014)
NIAID: EXPERIMENTAL CHIKUNGUNYA VACCINE
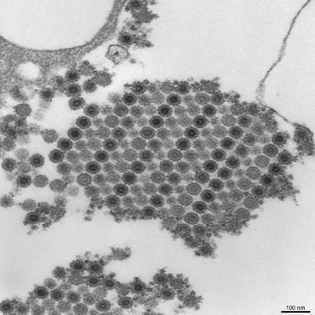
CYNTHIA GOLDSMITH, CDC
NIAID’s Vaccine Research Center (VRC) tested a promising experimental vaccine to prevent the mosquito-borne viral illness chikungunya. Above: This transmission electron micrograph (TEM) depicts numerous chikungunya virus particles, which are composed of a central dense core that is surrounded by a viral envelope. Each virion is approximately 50 nanometers in diameter.
An experimental vaccine to prevent the mosquito-borne viral illness chikungunya elicited neutralizing antibodies in all 25 adult volunteers who participated in a recent early-stage clinical trial conducted by NIAID’s Vaccine Research Center (VRC). Chikungunya infection is characterized by severe joint pain accompanied by headache and fever. There are currently no vaccines or specific drug treatments for chikungunya. First identified in East Africa in the early 1950s, the chikungunya virus has been documented in 40 countries; it appeared in the Western Hemisphere in late 2013. In 2010, VRC scientists and colleagues tested this candidate chikungunya vaccine in nonhuman primates. All of the immunized animals were protected from infection when later exposed to chikungunya virus. In the newly reported trial, 23 healthy volunteers each received three injections (two other volunteers received two injections) of vaccine at one of three different dosages (10, 20, or 40 micrograms) over 20 weeks. Antibody production was measured at multiple time points after each injection. Investigators detected chikungunya-neutralizing antibodies in all volunteers after the second injection, with a significant boost of neutralizing antibodies seen after the third injection. Vaccine-induced antibodies persisted in all volunteers, even those who received the lowest dosage, for at least 11 months after the final vaccination, suggesting that the vaccine could provide durable protection. (NIAID VRC authors: L.-J. Chang, K.A. Down, G.J. Nable, J.E. Ledgerwood, and others, Lancet DOI:10.1016/S0140-6736(14)61185-5)
NCI, NICHD: SUBCELLULAR IMAGING VISUALIZES BRAIN RECEPTORS
NCI and NICHD scientists have created high-resolution images of the glutamate receptor, a protein that plays a key role in neuronal signaling. The advance opens a new window to study protein interactions in cell membranes in exquisite detail. The scientists used an imaging technique called cryo-electron microscopy (cryo-EM), an emerging tool for obtaining protein structures in various states. Cryo-EM is a more versatile approach for obtaining protein structures than the commonly used method of X-ray crystallography, a process that requires scientists to force the protein to crystallize in a fixed shape.
The glutamate receptor serves as a channel to allow ions into the nerve cell, which induces nerves to send signals. The dysfunction of this receptor has been implicated in some types of cancer as well as in neurodegenerative and psychiatric disorders, including Parkinson disease and depression. Understanding how the ion channels operate could lead to the creation of medications that inhibit or enhance these receptor motions. (NCI authors: J.R. Meyerson, P. Rao, S. Subramaniam; NICHD authors: J. Kumar, S. Chittori, M.L. Mayer, Nature DOI:10.1038/nature13603)
NIA, NHLBI: SIX NEW GENETIC RISK FACTORS FOR PARKINSON DISEASE
Using data from some 18,000 patients, NIH scientists have identified more than two-dozen genetic risk factors involved in Parkinson disease, including six that had not been previously reported. The NIH researchers collaborated with multiple public and private organizations to collect and combine data from existing genome-wide association studies, which allow scientists to find common variants in the genetic codes of large groups of individuals. The combined data included approximately 13,708 Parkinson disease cases and 95,282 control subjects, all of European ancestry. The investigators identified potential genetic-risk variants, which increase the chances that a person may develop Parkinson disease. Their results suggested that the more variants a person has, the greater the risk, up to three times as high, for developing the disorder. Some of the newly identified genetic risk factors are thought to be involved with Gaucher disease, regulating inflammation and the nerve-cell chemical-messenger dopamine as well as alpha-synuclein, a protein that has been shown to accumulate in the brains of some people with Parkinson disease. Further research is needed to determine the roles of the variants identified in this study. (NIA authors: M.A. Nalls, D.G. Hernandez, M.F. Keller, S. Arepalli, C. Letson, C. Edsall1, H. Pliner, A.B. Singleton; NHLBI author: A.L. DeStefano, Nat Genet DOI:10.1038/ng3043)
NIAID: NASAL TEST TO DETECT PRION DISEASE
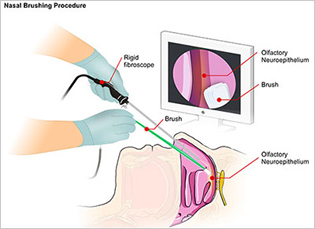
NIAID
NIAID and Italian scientists developed a promising nasal brush test that can rapidly and accurately diagnose Creutzfeldt-Jakob disease (CJD), an incurable and ultimately fatal neurodegenerative disorder. The involves the insertion of a rigid fiber-optic rhinoscope into the patient’s nasal cavity. A sterile brush is then inserted alongside the scope to collect olfactory neurons by gently rolling along the mucosal surface.
NIH and Italian scientists have developed a nasal brush test that can rapidly and accurately diagnose Creutzfeldt-Jakob disease (CJD), an incurable and ultimately fatal neurodegenerative disorder. CJD is a prion disease in which normally harmless prion protein molecules become abnormal and gather in clusters. The most common form, sporadic CJD, affects an estimated one in one million people annually worldwide. Other prion diseases include scrapie in sheep; chronic wasting disease in deer, elk, and moose; and bovine spongiform encephalopathy, or mad cow disease, in cattle.
Scientists have associated the accumulation of these clusters with tissue damage that leaves spongelike holes in the brain. Human prion diseases can be transmitted via medical procedures such as blood transfusions, transplants, and use of contaminated surgical instruments. Up to now, a definitive CJD diagnosis required testing brain tissue obtained after death or by biopsy in living patients. The new diagnostic test, which involves collecting olfactory neurons in the nasal cavity, would let doctors clearly differentiate prion diseases from other brain diseases and help to prevent the spread of prion disease. (NIAID authors: C.D. Orru, M. Bonginni, A.G. Hughson, B.R. Groveman, and B.Caughey, N Engl J Med 371:519–529, 2014)
NIAMS, NHLBI, NHGRI, NIDCD: GENE LINKED TO FATAL INFLAMMATORY DISEASE IN CHILDREN
NIH investigators have identified a gene that underlies a very rare but devastating autoinflammatory condition in children. Several existing drugs have shown therapeutic potential in laboratory studies, and one is currently being studied in children with the disease, which the researchers named SAVI, short for stimulator of interferon genes protein–associated vasculopathy with onset in infancy. (NIH authors: Y. Liu, A.A. Jesus, B. Marrero, R. Goldbach-Mansky, and others, N Engl J Med DOI:10.1056/NEJMoa1312625)
NEI, NCI: GENE CRITICAL TO THE EARLY DEVELOPMENT OF CILIA
NEI and NCI researchers have described the functions of a gene responsible for anchoring cilia, the sensory hairlike extensions present on almost every cell of the body. In mice without the gene Cc2d2a, cilia throughout the body failed to grow, and the mice died during the embryonic stage. The finding adds to an expanding body of knowledge about ciliopathies, a class of genetic disorders that result from defects in the structure or function of cilia. (NIH authors: S. Veleri, A. Swaroop, and others, Nat Commun 5:4207, 2014)
NCI: EXTREME OBESITY MAY SHORTEN LIFE EXPECTANCY UP TO 14 YEARS
Adults with extreme obesity have increased risks of dying at a young age from cancer and many other causes including heart disease, stroke, diabetes, and kidney and liver diseases, according to results of an analysis of data pooled from 20 large studies of people from three countries. The study, led by NCI researchers, found that people with extreme obesity had a dramatic reduction in life expectancy compared with people of normal weight. Extreme obesity is defined as a body mass index (BMI) of 40.0 or higher; normal weight is a BMI of 18.5-24.9. (NCI authors: C.M. Kitahara, P. Hartge, et al., PLoS Med 11:e1001673, 2014)
NHGRI, NCI, CIT, NHLBI: NEW GENETIC ASSOCIATION WITH CORONARY ARTERY DISEASE
Researchers at NHGRI, working with groups from NCI, NHLBI, and CIT, have found an innovative way to identify genes associated with coronary artery calcification by next-generation sequencing of RNA from subjects enrolled in the ClinSeq study, substantiated with DNA and protein evidence.
Results from this research suggest that the TREML4 gene is associated with coronary calcification. Subjects in the ClinSeq study and the Framingham Heart Study with higher coronary calcium scores had increased concentrations of the protein triggering receptor expressed on myeloid cells-like 4 (TREML4) in their blood. Sequencing also identified a genomic polymorphism within the TREML4 gene; people with the less-common allele in their genome were up to 6.5 times as likely to develop advanced amounts of coronary calcium compared with people without the allele. In addition, autopsy samples of atherosclerotic plaques from patients with coronary artery disease (CAD) showed high concentrations of TREML4 protein, whereas arteries with less advanced CAD did not have any TREML4.
As a complement to genome-wide association studies, the current integrative study of TREML4 and coronary calcification used consistent biological evidence at all stages of the experimental process (from RNA to DNA to protein) to connect the dots from candidate gene discovery to vascular malformation pathology. (NIH authors: S.K. Sen, L.G. Bieseker, and others, Am J Hum Genet 95:66–76, 2014)
NIDDK: UNDERSTANDING THE MOLECULAR BASIS OF A SICKLE-CELL DRUG
NIDDK researchers have identified a molecular basis for a key beneficial effect of the drug hydroxyurea in patients with sickle-cell disease (SCD). Hydroxyurea increases fetal hemoglobin concentrations in the red blood cells of patients with SCD, thus diluting the concentration of sickled red cells and so decreasing the tendency of red cells to block blood flow to tissues. The drug is the only one approved by FDA for treating SCD, and in many patients it reduces common complications such as severe pain and organ damage.
Since its formal approval as a SCD medication in 1998, doctors have prescribed hydroxyurea to patients without fully understanding how it works. Researchers had previously identified expression of the SAR1 gene as crucial to the drug’s ability to increase fetal hemoglobin concentrations in red blood cells, and more recent findings confirm this. In the latest study, suppression of SAR1 expression in red blood cell precursors prevented hydroxyurea from stimulating fetal hemoglobin production. Researchers conversely found that experimental overexpression of SAR1 activated a genotoxic stress pathway (which regulates DNA stability after exposure to carcinogens) and promoted fetal hemoglobin production. These findings suggest that molecular pathways involved in SAR1 expression may provide targets for designing new fetal hemoglobin–stimulating drugs that may be useful for the treatment of SCD and thalassemia. Additionally, the findings empower physicians to educate patients and their families on how hydroxyurea affects the body. (NIH authors: J. Zhou, K. Chin, W. Aerbajinai, C. Kumkhaek, H. Li, G.P. Rodgers, Blood 124:1146–1156, 2014)
NCATS: FIRST DRUG CANDIDATE ACQUIRED BY BIOPHARMACEUTICAL COMPANY
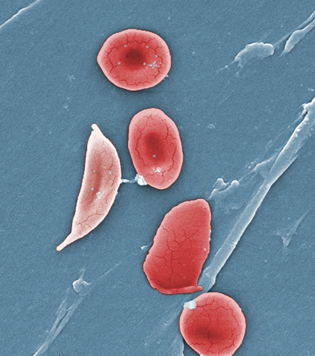
JANICE HANEY CARR, CDC
This digitally colorized scanning-electron micrograph revealed some of the comparative ultrastructural morphology between normal red blood cells (RBCs), and a sickle cell RBC (left) found in a blood specimen of an 18-year-old female patient with sickle-cell disease (SCD). NIDDK researchers have identified a molecular basis for a key beneficial effect of the drug hydroxyurea in patients with SCD. NCATS developed another SCD drug—Aes-103—which works by binding directly to hemoglobin and changing its structure, thereby reducing the sickling of RBCs. Aes-103 has been acquired by Baxter International’s BioScience business.
A drug candidate—Aes-103—developed by NCATS researchers and collaborators to treat sickle-cell disease (SCD), has been acquired by Baxter International’s BioScience business. Aes-103, the first specifically developed drug to target the underlying molecular mechanism of SCD, binds directly to hemoglobin and changes its structure, thereby reducing the sickling of red blood cells. Currently, the only FDA-approved drug to treat SCD is hydroxyurea, which not everyone responds to favorably. Baxter now will advance the Aes-103 clinical development activities required for regulatory approval and commercialization. This is the first time a company has acquired a drug candidate developed with NCATS’s Therapeutics for Rare and Neglected Diseases (TRND) program resources. To read more about NCATS and its TRND program, visit http://www.ncats.nih.gov/trnd.html.
This page was last updated on Tuesday, April 26, 2022
