Research Festival: Selected Symposia
Highlights
Couldn’t get to all the symposia sessions at the Research Festival or you want a refresher on what you heard and saw? Here’s a sampling of what went on: “Sugar, Sugar” (about glycobiology research); “Pain, Pain, Pain”; “Aging Molecularly”; “Neurogenetic Analysis”; “T Cells Through Old Age”; “Using RNAi to Discover Genes”; “Tricking Viruses into Treating Disease”; “Optogenetic Manipulation of Neural Circuits and Behavior”; “Seriously Studying Stem Cells”; “Breast Is Best: Infant Dietary Guidelines”; and “Natural Born (Cancer) Killers.”
Sugar, Sugar
When Julie Andrews sang, “Just a spoonful of sugar makes the medicine go down” in the 1964 Disney film Mary Poppins, could she have inspired budding scientists to one day explore the therapeutic value of sugars? After all, the field of glycobiology, which is the study of complex sugar molecules, or glycans, was launched more than 20 years after the movie came out. Today, scientists at NIH and elsewhere hope that a better understanding of glycobiology can be leveraged for therapeutic and diagnostic purposes and may even lead to the development of more effective vaccines.
Malfunction of a nuclear-cytoplasmic signaling pathway involving the simple glycan O-linked N-acetylglucosamine (O-GlcNAc) is involved in immunity-linked human diseases ranging from diabetes to lupus, explained John Hanover (NIDDK), who has identified and characterized the genes encoding the enzymes of O-GlcNAc cycling.
The intracellular carbohydrate O-GlcNAc plays an important role in the innate immune response of the nematode Caenorhabditis elegans and may hold the key to understanding the immune response to pathogens, according to FARE Winner Michelle Bond (NIDDK). Loss of the enzymes governing the addition and removal of O-GlcNAc triggers stress- and immune-responsive genes.
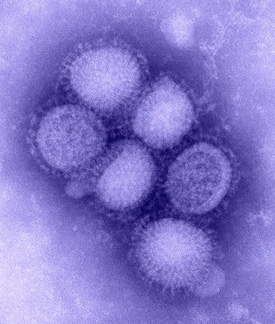
JANICE CARR, CDC
Flu vaccines may be improved once scientists have a better handle on glycobiology and glycans. Seema Lakdawala (NIAID) is researching the role of glycans in the 2009 pandemic H1N1 flu virus and found that the receptor specificity of H1N1 is more complex than previously thought. Shown: Transmission electron micrograph of the H1N1 influenza virus.
In the vaccine arena, Joanna Kubler-Kielb (NICHD) described the development of vaccine candidates consisting of carbohydrate antigens bound protein carriers in order to protect against infections caused by Gram-negative bacteria genera such as Bordetella and Brucella. Jeffrey Gildersleeve (NCI), whose lab uses carbohydrate antigen microarrays to study immune responses to vaccines, has identified new biomarkers for predicting clinical responses to cancer vaccine therapy.
Even flu vaccines may be improved once scientists have a better handle on glycobiology and glycans. Seema Lakdawala (NIAID), who is researching the role of glycans in the 2009 pandemic H1N1 flu virus, found that the receptor specificity of H1N1 is more complex than previously thought. Evidence suggests that flu viruses infect host cells by binding to a wide range of acidic sugars.
From the 2013 Research Festival’s session “The glycobiology of immunity and infection,” chaired by Pamela Marino (NIGMS), and held on November 8, 2013.
Pain, Pain, Pain
Increasingly, researchers across NIH are tackling chronic pain in an attempt to understand its mechanisms and how to treat or prevent it. That news should come as welcome relief to the 100 million Americans who suffer from debilitating, persistent pain, which may be triggered by physical trauma; may be linked to arthritis, cancer, or other conditions; or may have no apparent cause at all.
Take fibromyalgia, a disorder characterized by widespread musculoskeletal pain, abnormal pain processing, fatigue, sleep disturbances, memory problems, psychological distress, and memory and mood issues. No one knows for sure what causes it, but it affects three million to six million Americans. NINR’s Brian Walitt outlined the ailment’s history and discussed current debates on whether fibromyalgia is a product of disordered neural pain processing or a result of psychological and cultural factors.
NCCAM’s Scientific Director M. Catherine Bushnell has identified one effect of fibromyalgia-associated chronic pain on the brain: Magnetic resonance imaging studies have shown that fibromyalgia patients have reduced gray matter in brain regions related to pain processing. Bushnell has also investigated links between other chronic-pain conditions and the brain. She found that successful surgical treatment of chronic lower-back pain slowly reverses brain atrophy, and long-time yoga practitioners have higher gray-matter density in pain tolerance regions of the brain. Her work suggests that neuroprotective approaches may benefit chronic-pain patients.
Even diet may play a role in helping to control chronic pain. Many pain-signaling compounds are derived from dietary fats, for instance. Christopher Ramsden (NIAAA) and extramural colleagues conducted a clinical trial to assess how dietary modification could decrease the incessant pain of chronic headaches. They hypothesized that pain signals could be decreased by altering dietary proportions of omega-6 and omega-3 fatty acids. Indeed, these dietary modifications altered pain-signaling compounds and reduced headaches. The researchers are now conducting a larger trial to expand upon these encouraging results.
New insights into the mechanisms of nociception (perception of pain) came from NIDCR’s Mark Hoon and FARE Award Winner Leah Pogorzala, who works in his lab. They described their studies of sensory neural circuits involved in thermosensation and itch; those pathways overlap with pain signaling. Pogorzala has identified discrete neuron populations that independently respond to hot and cold stimulation; Hoon explained the lab’s delineation of neuronal pathways for itch responses. Their findings are paving the way to a greater understanding of sensory and pain perception.
References
- Institute of Medicine Report from the Committee on Advancing Pain Research, Care, and Education: Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research (Washington, D.C.: National Academies Press, 2011).
- F. Wolfe and B. Walitt, “Culture, science and the changing nature of fibromyalgia,” Nat Rev Rheumatol 9:751–755, 2013.
- A. Kuchinad, P. Schweinhardt, D.A. Seminowicz, P.B. Wood, B.A. Chizh, and M.C, Bushnell, “Accelerated brain gray matter loss in fibromyalgia patients: Premature aging of the brain?” J Neurosci 27:4004–4007, 2007.
- D.A. Seminowicz, T.H. Wideman, L. Naso, Z. Hatami-Khoroushahi, S. Fallatah, M.A. Ware, et al., “Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function,” J Neurosci 31:7540–7550, 2011.
- C. Villemure, M. Čeko1, V.A. Cotton, and M.C. Bushnell, “Insular cortex mediates increased pain tolerance in yoga practitioners,” Cereb Cortex DOI:10.1093/cercor/bht124, 2013.
- C.E. Ramsden, K.R. Faurot, D. Zamora, C.M. Suchindran, B.A. MacIntosh, S. Gaylord, et al., “Targeted alteration of dietary n-3 and n-6 fatty acids for the treatment of chronic headaches: A randomized trial,” Pain 154:2441–2451, 2013.
- L.A. Pogorzala, S.K. Mishra, and M.A. Hoon, “The cellular code for mammalian thermosensation,” J Neurosci 33:5533–5541, 2013.
- S.K. Mishra and M.A. Hoon, “The cells and circuitry for itch responses in mice,” Science 340:968–971, 2013.
From the 2013 Research Festival’s session “Pain and nociception: From patients to molecules,” chaired by Mark Hoon (NIDCR), and held on November 6, 2013.
Aging Molecularly
The seemingly unstoppable process of aging is the major biological risk factor for many chronic diseases including cancer, heart disease, and type 2 diabetes. The discovery that aging not only is an accumulation of random damage, but also is governed by molecular mechanisms that are susceptible to manipulation and intervention, suggests that trans-NIH aging research could potentially address multiple chronic diseases all at once.

JUDITH STOFFER
Aging has been linked to increased obesity, declining mitochondrial function, and decreased thermogenesis, indicating a switch from “energy-using” to “energy-storing” mode. Mitochondria (shown above) are the cells’ power plants. Damaged mitochondria accumulate during aging and are marked for elimination by a self-eating process called mitophagy.
One of the important areas in this field is cellular energy sensing and balance. Aging has been linked to increased obesity, declining mitochondrial function, and decreased thermogenesis (heat production), indicating a switch from “energy-using” to “energy-storing” mode. Reversing this energy mode through caloric restriction has been shown to extend lifespan. How the cell senses and responds to its energy state involves the sirtuin proteins, which alter chromatin organization and gene-expression programs.
Jay Chung (NHLBI) presented data showing a new way the mammalian sirtuin 1 (SIRT1) senses the energy status of the cell: It has an adenosine triphosphate (ATP)–dependent peptide switch that is “on” during energy depletion, whereas excess ATP binds this switch region and blocks SIRT1 activity in the “off” state. Global chromatin organization by SIRT1 is also altered by DNA damage, causing global gene-expression changes similar to those found in aged cells. New work from Philipp Oberdoerffer (NCI) suggests that DNA damage signals chromatin structure to affect the way cells repair DNA damage.
Studying the cellular changes that occur upon caloric restriction will help scientists understand its mechanism, so the lab of Jennifer Lippincott-Schwartz (NICHD) examined cell components in nutrient-deprived yeast. They found that cells display an autophagic (“self-eating”) response, called lipophagy, involving digestion of intracellular lipids. This lipophagy, they report, requires fusion of individual mitochondria into a tubular network, suggesting that lipid recycling and mitochondrial function are amplified for an efficient response to starvation.
Damaged mitochondria, in contrast, accumulate during aging and are marked for elimination by a different self-eating process called mitophagy. This process requires the parkin protein, whose gene, PARK2, is mutated in some autosomal recessive forms of Parkinson disease. Chiu-Hui Huang (NINDS) discussed her team’s new mouse model for Parkinson disease, which required more than simply eliminating endogenous parkin. To see Parkinson-like effects in their knockout mice, they had to mimic age-dependent mitochondrial DNA damage accumulation with a second mutation in a mitochondrial DNA polymerase.
Age-related decreases in mitochondria-rich, heat-producing brown fat relative to lipid-storing white fat is linked to the onset of obesity, type 2 diabetes, and other disorders. FARE Award winner Lingyan Xu (NIDDK) identified a role for forkhead box (FOX) family transcription factors in brown versus white adipocyte balance. Xu found that mice deficient in the protein Foxa3 have increased energy expenditure and thermogenesis due to the “browning” of white adipose tissue. These mice were protected against age-related obesity and insulin resistance, suggesting that Foxa3 ablation could be a novel therapeutic target to treat aging-associated metabolic disorders. As the links between metabolism and aging continue to emerge, the ancient Hippocratic notion of food as medicine may unlock a modern fountain of youth.
From the Research Festival session “Molecular Mechanisms of Aging,” chaired by Felipe Sierra (NIA), sponsored by the GeroScience Interest Group, and held on November 8, 2013.
Neurogenetic Analysis of Behavioral Circuits

NIH (iSTOCK PHOTO)
NIH researchers are mapping brain function to determine how neural circuits control behavior and how disruptions in brain function occur in diseases such as schizophrenia and autism.
New technological advances are revolutionizing our understanding of the human brain and may provide clues to treating, preventing, and curing brain disorders and diseases. NIH researchers with expertise in genetics, physiology, and microscopy are collaborating to map brain function in hopes of discovering how neural circuits control behavior and of determining how disruptions in brain function occur in such diseases as schizophrenia and autism.
Chi-Hon Lee (NICHD) combines behavioral, imaging, and molecular genetic approaches to study visual circuit development and function in Drosophilia. By selectively inactivating and restoring the synaptic activity of different types of neurons, Lee’s lab is trying to dissect color-vision circuits that drive color learning. Another Drosophila researcher, Susan Harbison (NHLBI), investigates the genetic networks underlying sleep and their interactions with the environment. She uses high-throughput genotyping to observe heritable differences in sleep patterns and has demonstrated how significant changes in sleep occur in just a few generations. She is also investigating the manner in which DNA polymorphisms and variation in transcript, protein, and metabolite concentrations lead to differences in sleep.
Jeffrey Smith (NINDS) uses optogenetics to unravel the brain networks responsible for breathing. Optogenetics involves taking a gene for light-activated membrane channels targeted to a single neuron type, inserting it into a mouse genome, and then shining a light into the brain to turn those neurons on or off. He is also using whole-cell patch-clamp recording techniques to study the biophysical and synaptic properties of respiratory circuits in the brainstem and to explain the neurogenesis of respiratory movements.
To understand how neuronal circuits develop, Tudor Badea (NEI) combines molecular genetics tools—conditional gene ablation and reporter-gene replacement—to label individual neurons in the retina, visualize the morphology and connectivity of specific cell populations, and study their role in the visual system.
Fumihito Ono (NIAAA) wants to answer fundamental questions about neuromuscular development and function. In particular, he uses the genetic tools of locomotor mutants, cell-specific transgenics, and a chemically inducible gene-expression system to see how neuromuscular synapses form in zebrafish.
In other research exploring synapses, FARE Award Winner Carmelo Sgobio (NIA) studies presynaptic calcium modulation in midbrain neuron terminals. He uses tetracycline-controlled transcriptional activation to reveal how synaptic transmission at nerve terminals is mediated and regulated.
From the 2013 Research Festival session “Neurogenetic analysis of behavioral circuits,” chaired by Harold Burgess (NICHD) and Kevin Briggman (NINDS), and held on November 7, 2013.
T Cells Through Old Age
T cells play an important role in the immune system’s response to pathogens and tumor cells. However, to prevent autoimmunity, the body’s T-cell population must be monitored to ensure proper development and homeostasis. Several labs at NIH investigate the molecular pathways that regulate normal T-cell development as well as the challenges to T-cell homeostasis brought on by cancer chemotherapies and the natural aging process.
To minimize autoimmunity, T cells expressing self-reactive T-cell antigen receptors (TCR) undergo programmed cell death (apoptosis). Paul Love (NICHD) created knock-in mice in which TCR signaling is significantly reduced, disrupting apoptosis. Surprisingly, the mice failed to develop autoimmune diseases, leading Love and his colleagues to suggest the existence of a compensatory pathway for immune tolerance that results in the increased development of regulatory T cells (Tregs). Xuguang Tai (NCI) has identified the pro-apoptotic and pro-survival signaling mechanisms by which a transcription factor called forkhead box P3 regulates the development of regulatory Tregs that help prevent autoimmune disease.
Nan-ping Weng (NIA) has developed a next-generation sequencing method to characterize the diversity of TCRs. His group discovered that CD4 T cells have twice as many unique TCRs as CD8 T cells do, and the group observed age-related alteration of TCR repertoire in people 45 to 65 years old, but not in people 75 to 94 years old.
Joy Williams (NCI) reported that the development of medullary thymic epithelial cells (mTEC), which depends on the presence of a type of thymocyte—called single positive (SP) thymocytes and eliminates autoreactive T cells—relies on the activation of a particular pathway. When this pathway is activated, mTECs can develop even in the absence of SP thymocytes.
Frank Flomerfelt (NCI) discovered that the thymus, brain, and testes-associated gene (Tbata) regulates thymic epithelial cell proliferation and thymus size by blocking a pathway implicated in cell-cycle progression. His group observed that Tbata expression increases with age and that deletion of Tbata results in a larger thymus later in life.
Treating cancer often leads to immunosuppression. FARE award winner Liat Izhak (NCI) studies the cross-modulation among various immunosuppressive mechanisms driven by Tregs and natural killer T cells.
From the 2013 Research Festival’s session “T-cell development, aging, and thymus regeneration,” co-chaired by Paul Love (NICHD) and Nan-ping Weng (NIA), and held on November 8, 2013.
Using RNAi to Discover Genes

CARLEEN KLUMPP, NCATS
Gene silencing through RNA interference (RNAi) has become a standard laboratory tool for assessing gene function, but to make the jump to discovery on a large scale most intramural scientists need help. NCATS’s Trans-NIH RNAi Screening Facility has automated systems, like the one shown here, that perform genome-wide RNAi screening.
Gene silencing through RNA interference (RNAi) has become a standard laboratory tool for assessing gene function, but to make the jump to discovery on a large scale most intramural scientists need help. This is where the NCATS’s Trans-NIH RNAi Screening Facility comes in: Using genome-wide RNAi screening, intramural investigators can make exciting discoveries about the role of genes and pathways associated with human diseases. Scott Martin, the leader of the Trans-NIH RNAi Screening Facility, outlined how his team goes about helping intramural scientists to develop and perform genome-wide RNAi screens. RNAi studies on this scale also need careful data analysis. Gene Buehler (NCATS) uses computational analysis to eliminate false-positive results and to identify disease-associated genes. His work has been critical to the success of the many screening projects the NCATS team has helped develop.
Lesley Kane (NINDS) and colleagues are using genome-wide RNAi screening to better understand the parkin-mediated mitochondria quality-control mechanism. Mutations in the gene PARK2, which codes for parkin, are known to cause Parkinson disease. Using a cell-based, high-content assay of parkin translocation, Kane’s group has identified several new genes involved in this process and recently published its findings in Nature (Nature 504:291–295, 2013). doi:10.1038/nature12748.
Another NINDS group, led by Barrington Burnett, uses genome-wide RNAi screening to identify genetic modifiers of spinal muscular atrophy (SMA). SMA is a debilitating neurological disease that leads to muscle weakness and wasting and is caused by a deficiency of the survival motor neuron (SMN) protein. The screen identified a number of genes—including some associated with splicing and protein stability—that affect SMN protein concentrations.
Matthew Hall (NCI) gave a presentation on a microRNA functional genomic screen that identifies novel regulators of proteins that mediate resistance to the chemotherapy drug cisplatin. Hall has identified several microRNAs and their targets that are responsible for tumors becoming resistant to cisplatin. His group is currently following up this work with a whole-genome RNAi screen of protein-encoding genes.
The audience was also treated to a presentation by FARE Award Winner Tuan Tran (NIAID). Tran described a novel method for assessing malaria risk: Looking for naturally acquired antibodies specific for the Plasmodium falciparum parasite protein PfRH5, whose presence predicted protection from malaria in a cohort of children and adults in Mali.
From the 2013 Research Festival’s session on “Genes and pathway discovery in the context of human diseases,” chaired by Scott Martin (NCATS) and Natasha Caplen (NCI), and held on November 7, 2013.
Tricking Viruses into Treating Disease
Viruses infect host cells by tricking them into expressing viral genes, but scientists can trick viruses in return by using them to express health-promoting genes to treat inborn errors of metabolism, immunodeficiency, and cancer. Fabio Candotti (NHGRI) is evaluating retroviral vectors that express the enzyme adenosine deaminase to treat patients with severe combined immunodeficiency. To develop treatment for a fatal neurodegenerative disorder called Menkes disease, Marie Reine Haddad (NICHD), is testing whether the injection of adeno-associated viral vectors into the cerebrospinal fluid of mice models of Menkes disease restores brain expression of the copper transporter gene ATP7A in the brain, extends the survival of the mice, and normalizes neurological function.
Wenqin Xu (NIMH) described his work with a novel retrovirus isolated from koalas that may one day provide clues to viable alternatives for retroviral mediated gene therapy. The koala retrovirus, which uses the thiamine transporter as a receptor, can be used to map regions within this class of virus that can be modified to use different receptors (see article in the November-December 2013 NIH Catalyst).

However, there can be pitfalls in viral gene therapy, such as unwanted side effects, cautioned Randy Chandler (NHGRI). For example, he found that gene therapy (adeno-associated viral expression of the methylmalonyl-Co-enzyme A mutase gene) restored normal development in mice with the genetic metabolic disorder methylmalonic acidemia, but it occasionally caused liver cancer, too.
Still, NIH scientists have had a lot of success in tricking viruses into being helpful. Steven Feldman (NCI-CCR) has participated in several clinical trials in which T cells reprogrammed with retroviral vectors caused remission in several types of cancer. And FARE award winner Gabriel Parra (NIAID) is developing a vaccine that provides protection against multiple strains of norovirus, one cause of viral gastroenteritis.
From the 2013 Research Festival’s session “Therapeutic viral-mediated gene delivery in the 21st century,” chaired by Maribeth Eiden (NIMH), and held on November 8, 2013.
Optogenetic Manipulation of Neural Circuits and Behavior
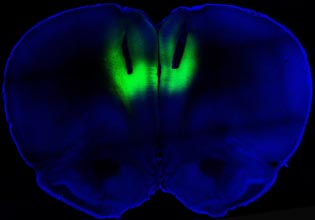
BILLY CHEN AND ANTONELLO BONCI, NIDA
Optogenetics is a revolutionary new technique in which light-activated proteins selectively control the activity of specific cell types in the brain. Mice that are engineered to carry light-activated neurons in the neural pathways being studied are fitted with optic fibers to transmit harmless pulses of laser light. This image, from the NIH Director’s Blog, shows how optogenetic stimulation lights up the prelimbic cortex in cocaine-addicted rats, blocking their desire for the narcotic.
Optogenetics is a revolutionary new technique in neuroscience in which light-activated proteins selectively control the activity of specific cell types in the brain. A small optical fiber is implanted into a mouse brain and, as it is turned on and off, it stimulates the neurons in which genes express light-activated proteins. Because researchers cause expression of light-gated proteins in specific neurons, photostimulation activates only neurons that express the desired protein channel, allowing for the study of specific neuronal pathways.
Michael Krashes (NIDDK) and Yeka Aponte (NIDA), both recently hired tenure-track investigators, published seminal research papers during their postdocs about using optogenetics and pharmacogenetics to demonstrate the role of certain neurons in regulating feeding behavior. Krashes’s discoveries may one day help curb obesity as he expands on his previous work untangling the complex neuronal pathways controlling feeding. Aponte will study neuronal pathways that regulate goal-oriented behavior and how these behaviors are disrupted in drug addiction and eating disorders. Her findings may lead to better treatments for people struggling with drug abuse and serious disturbances of eating behavior.
Olena Bukalo (NIAAA) uses optogenetics to better understand the complex neuronal circuitry underlying many anxiety disorders. She is interested in the interactions between the ventromedial prefrontal cortex (which controls decision-making and processing risk and fear) and the amygdala (which processes memory and emotion) with the goal of developing better treatments for anxiety disorders such as post-traumatic stress disorder.
FARE Award Winner Anton Ilango Micheal (NIDA) is studying how the dopamine system regulates reward and aversion behavior. Micheal generated mice expressing light-gated ion channels in two midbrain regions—the ventral tegmental area (VTA) and the substantia nigra pars compacta (SNc). Previously, the VTA was thought to be solely responsible for reward and aversion whereas the SNc was linked with motor and cognitive control. He found, however, that the excitation and inhibition of dopamine neurons in the SNc can elicit reward and aversion behaviors.
Joshua Puhl (NINDS) uses an ex vivo technique to study locomotor behavior and is interested in how the central pattern generators (CPGs), located in the spinal cord, function during locomotor-like activity. CPGs are neural networks that produce rhythmic patterned outputs without sensory feedback, such as those involved in respiration, swallowing, and locomotion. Locomotor CPGs within the spinal cord allow us to walk with limited cognitive input from the brain. Pulh found that suppression of motor-neuron impulses affect pattern generation within CPGs. His findings not only shed light on how CPGs function but also may aid in developing therapies for disabled individuals.
From the 2013 Research Festival session “Optogenetic manipulation of neural circuits and behavior,” chaired by Lex Kravitz (NIDDK), and held on November 6, 2013.
Seriously Studying Stem Cells
One day, scientists may be able to create living, functional tissue from stem cells. Stem cells have potential for therapeutic use in regenerative medicine and as targets for anticancer therapies. To understand the molecular mechanisms that determine stem-cell behavior, Steven Hou (NCI) uses adult fruit flies of the genus Drosophila as a model. He has found a unique mechanism involving chromatin-remodeling factors that regulates how intestinal stem cells differentiate.
Scientists also rely on mouse models for stem-cell research. Wendy Knosp (NIDCR) is exploring how stem cells develop into functional salivary glands. She hopes her findings will lead to a procedure that will help people whose salivary glands have been damaged by radiation therapy, certain medications, or hereditary conditions. Terry Yamaguchi (NCI) is interested in how protein-signaling pathways regulate less specialized stem-cells types that become more specialized. He has identified genes—many of them common in intestinal tumors—that help maintain and form stem cells.
Isaac Brownell (NCI) and FARE Winner Ying Xiao (NCI), who also work with mice, study signaling pathways that regulate the development and maintenance of skin as well as the changes that occur during the formation of skin cancer. Brownell has demonstrated that a perineural niche helps to regulate the microenvironment of hair follicle stem cells. Xiao has found that blood vessels form a perivascular stem-cell niche in developing and regenerating hair follicles.
Some scientists, such as Sohyun Ahn (NICHD), focus on stem cells in the brain. Ahn described how changes in the sonic hedgehog signaling pathway affect the transition of embryonic neural stem cells to postnatal stem cells involved in neurogenesis in mature brain tissue.
From the 2013 Research Festival’s session “Stem Cells in Development and Diseases,” chaired by Steven Hou (NCI), and held on November 7, 2013.
Breast is Still Best: Infant Dietary Guidelines Get an Update for the 21st Century
Experts advise mothers to breastfeed their babies exclusively (that is, no complementary solid food or other beverages) for the first six months of life, yet nationwide, only 13 percent of mothers do so. According to Tonse Raju (NICHD), this percentage is low compared with other industrialized nations. Breastfeeding has been linked to decreases in infant health problems such as ear infections and poor development of the gastrointestinal system. Improving breastfeeding rates in this country to the 90 percent levels seen in many Scandinavian countries could save $19 billion health-care dollars annually.

“Issues like infant feeding choices are complex and involve interactions between maternal and infant biology, and the social-cultural context,” said Daniel Raiten (NICHD). They “need to be addressed with a systems approach.” Rosalind King (NICHD) stressed that successful continuation of breastfeeding over many months requires support from family members, workplaces, and the community.
Christine Rogers (NICHD) discussed the lack of breastfeeding imagery on infant products and social media sites. Increasing exposure to breastfeeding, she noted, could have a positive effect on breastfeeding behavior in the United States.
But “infant feeding must be more than a matter of advocacy,” said Raiten. “Fundamentally, we need the evidence to support best practices.” That knowledge is still evolving: Our understanding of the health consequences of dietary practices for infants older than six months—duration of breastfeeding, incorporation of complementary foods, and introduction to allergens—is still based more on conventional wisdom than hard science.
Comprehensive study of the nutrients and bioactives (molecules in food with both nutritional value and biological activity) in human milk could help provide guidance on nutritious, safe alternatives for the many mothers who, for a myriad of reasons, cannot breastfeed. The leading public-health effort to provide evidence-based advice about diet and health is the Dietary Guidelines for Americans (DGA), a partnership between HHS and USDA. To date, the DGA only cover children aged two and up.
Recently, NICHD has been leading the “B-24 project,” a collaboration between the two agencies to evaluate evidence to support the inclusion of infants and children from birth to 24 months of age in the DGA. It appears that, in large part due to NIH initiatives, breastfeeding is now getting the physical and intellectual support to document its superiority.
FARE Award winner Stefanie Hinkle (NICHD) also described her work showing an association between parity and birth weight in a longitudinal consecutive pregnancy study. This association was, until now, merely a general observation, but now is rooted in science. Her findings could help guide future clinical policy and epidemiological studies.
From the 2013 NIH Research Festival’s Session “One million babies are not breastfed each year: Perspectives on breastfeeding and infant nutrition,” chaired by Tonse Raju (NICHD), sponsored by the Breastfeeding and Human Lactation Scientific Interest Group, and held on November 7, 2013.
Natural Born (Cancer) Killers
Natural products—chemical substances produced by living organisms—have long been exploited as sources of medical drugs. The National Cancer Institute (NCI) boasts one of the world’s largest repositories of natural products, comprising more than 250,000 samples of mostly marine invertebrates, plants, fungi, and bacteria. NCI investigators and their collaborators routinely perform high-throughput screens of this collection to locate pharmacologically distinct compounds to combat cancer, human immunodeficiency virus (HIV), and AIDS.
Curtis Henrich (NCI) has screened the natural-products library to identify compounds that can enhance the cancer-cell-killing ability of the tumor necrosis factor–related apoptosis-inducing ligand (TRAIL). Using a TRAIL-resistant renal carcinoma cell line, his group, in collaboration with Thomas Sayers (NCI), has discovered a highly effective partner for TRAIL—a secondary metabolite of Physalis peruviana, a South American relative of the tomato plant.
Another investigator targeting kidney cancer with natural products is John Beutler (NCI). Beutler used NCI-60 tumor cell lines to identify a compound from the bark of Phyllanthus engleri, a tree native to Tanzania. The chemical kills cancer cells with a one-two punch: It makes them become addicted to glucose and then starves them by disrupting the glucose pathway.
High-throughput screening also helped Kirk Gustafson (NCI) find a chemical extract from the wooly sunflower, Eriophyllum lanatum, that prevents the degradation of programmed cell-death protein 4, a tumor suppressor protein.
James Inglese’s lab (NCATS)focuses on improving the biological fidelity and information content of high-throughput screening assays to test large libraries of natural-product extracts and has identified novel antimalarials.
Although research points to the effectiveness of resveratrol—an active ingredient in red wine—as an anticancer agent, its clinical use has been hindered by the lack of biomarkers to indicate its efficacy. Shakir Saud (NCI) reported the discovery of several biomarkers—including secondary bile acids—for monitoring resveratrol’s activity.
FARE Award Winner Geoffrey Vargish (NICHD) discussed recent findings that mice prenatally exposed to exogenous cannabinoids showed a significant, long-term deficiency in a specific class of inhibitory neurons that could precipitate cognitive defects and mood disorders.
From the 2013 Research Festival’s session “Advances in natural products research,” chaired by John Williamson (NCCAM), and held on November 6, 2013.
FARE stands for Fellows Award for Research Excellence
This page was last updated on Wednesday, April 27, 2022
