Research Briefs
NIDDK: DISRUPTING THE INTEGRITY OF THE SKIN
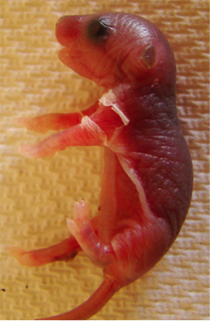
M. LAURA ALLENDE AND LAURA SIPE, NIDDK
The peeling skin on the forelimb of this four-day-old mouse is caused by a missing gene that controls the concentrations of a lipid molecule called sphingosine-1-phosphate.
Psoriasis and other skin diseases may be caused by disturbances in the balance between the growth and the differentiation of keratinocytes, the major cell type in the outer layer of skin. NIDDK researchers have found that a lipid molecule called sphingosine-1-phosphate (S1P) plays a key role in controlling the differentiation of keratinocytes. Mice missing the gene controlling intracellular S1P concentrations suffered from stunted growth and thickened skin that was prone to peeling within the first few days of life. Most of the mutant mice died, but the ones that survived had skin abnormalities—their keratinocytes had high concentrations of S1P, which enhanced keratinocyte differentiation. The findings suggest that manipulating S1P concentrations may be a way to alter the abnormal growth and differentiation of keratinocytes. The researchers say that one possible treatment for psoriasis—a disorder in which there is a hyperproliferation of keratinocytes—might be to increase S1P concentrations. (NIDDK authors: M.L. Allende, L.M. Sipe, G. Tuymetova, K.L. Wilson-Henjum, W. Chen, and R.L. Proia; J Biol Chem 288:18381–18391, 2013)
NIDCR: WHAT TRIGGERS ITCHING?
“What is an itch?” is a question that five-year-olds have used to stump Ph.D.s for years. Now, thanks to two scientists at NIDCR, we are closer to an answer. Itching was once thought to be a low-level form of pain, but the NIDCR scientists determined that a neurotransmitter called natriuretic polypeptide b (Nppb) is released in the spinal cord and carries the sensation of itch to the brain. They showed that mice lacking either Nppb or cells expressing its receptor in the spinal cord did not itch when administered itching agents, but were still sensitive to pain. Furthermore, an injection of Nppb led to the increased production of a previously identified itching molecule, the gastrin release peptide. So, though we do not yet have a complete answer to the itching question, the NIDCR scientists suspect that blocking Nppb might one day be a cure for chronic itching conditions such as eczema and psoriasis. (NIDCR authors: S.K. Mishra and M.A. Hoon; Science 340:968–971, 2013)
NICHD: WOMEN’S BRAINS ARE HARDWIRED TO FOCUS ON INFANT’S CRIES
Mothers have long suspected that women’s brains are hardwired to respond to the cries of a hungry infant. After all, a baby’s survival depends on being fed, and in nature, it’s females who need to respond. An NICHD researcher, in collaboration with other scientists, has shown that women’s brains do indeed react differently than men’s to a baby’s cries. The 18 people in the study (nine men and nine women) were told to let their minds wander as they listened to a 15-minute recording of white noise interspersed with the sounds of an infant crying. Functional magnetic imaging scans of the participants’ brains showed that, in the women, patterns of brain activity abruptly switched to an attentive mode when they heard the infant cries, whereas the men’s brains remained in the resting state. The results were the same regardless of whether the participants were parents or nonparents. Such studies documenting the brain activity patterns of adults represent the first stages of neuroscience research to understand how adults relate to and care for infants. (NICHD author: M. Bornstein, NeuroReport 24:142–146, 2013)
NIAAA: ANTI-SMOKING MEDICATION SHOWS PROMISE FOR TREATING ALCOHOL DEPENDENCE
Alcohol dependence is a chronic disease that includes symptoms such as craving, loss of control over drinking, withdrawal symptoms after stopping drinking, and tolerance (the need to drink greater amounts of alcohol to feel the same effect). NIAAA researchers, in collaboration with clinical investigators at other institutions, found that varenicline (marketed under the name Chantix), approved in 2006 to help people stop smoking, significantly reduced alcohol consumption and craving among 200 alcohol-dependent adults. Varenicline may work by partially stimulating receptors for nicotinic acetylcholine, a promising molecular target implicated in both nicotine and alcohol disorders. This hypothesis was supported by early animal studies, which showed that varenicline decreased alcohol consumption. The researchers conclude that longer treatment with varenicline and follow-up assessments to determine whether there are sustained effects would be a valuable next step in the development of this medication for alcohol problems. [NIAAA authors: R. Litten, J. Fertig, D. Falk, and M. Ryan; J Addict Med DOI:10.1097/ADM.0b013e31829623f4 (2013)]
NIAID: HOW HIV KILLS IMMUNE CELLS
Untreated human immunodeficiency virus (HIV) infection destroys a person’s immune system by killing infection-fighting cells, but precisely when and how HIV wreaks this destruction has been a mystery until now. New research by NIAID scientists revealed how HIV triggers a signal telling an infected immune cell to die. This finding has implications for preserving the immune systems of HIV-infected individuals. HIV replicates inside infection-fighting human immune cells called CD4+ T cells through complex processes that include inserting its genes into cellular DNA. The scientists determined that during this integration step, a cellular enzyme called DNA-dependent protein kinase (DNA-PK) becomes activated. DNA-PK normally coordinates the repair of simultaneous breaks in both DNA strands. As HIV integrates its genes into cellular DNA, single-stranded breaks occur where viral and cellular DNA meet. Nevertheless, the scientists discovered, the DNA breaks during HIV integration surprisingly activate DNA-PK, which then performs an unusually destructive action: It elicits a signal that causes the CD4+ T cell to die. The cells that succumb to this death signal are the very ones mobilized to fight the infection.
These new findings suggest that treating HIV-infected individuals with drugs that block early steps of viral replication—up to and including activation of DNA-PK and integration—not only could prevent viral replication, but also may improve CD4+ T cell survival and immune function. The findings also may shed light on how reservoirs of resting HIV-infected cells develop and may aid efforts to eliminate these sites of persistent infection. (NIAID authors: A. Cooper, M. Garcıa, C. Petrovas, T. Yamamoto, R.A. Koup, and G.J. Nabel; Nature 498:376–379, 2013)
NCI, NIAMS, CC, OD: LINK BETWEEN ALLERGIC AND AUTOIMMUNE DISEASES
NIH scientists and colleagues in Qatar and Japan have discovered that a gene called BACH2 may play a central role in the development of diverse allergic and autoimmune diseases, such as multiple sclerosis, asthma, Crohn's disease, celiac disease, and type 1 diabetes. The results of previous research had shown that people with minor variations in the BACH2 gene often develop allergic or autoimmune diseases, and that a common factor in these diseases is a compromised immune system. In this study in mice, the Bach2 gene was found to be a critical regulator of the immune system’s reactivity. The finding that a single component of the immune system plays such a broad role in regulating immune function may explain why people with allergic and autoimmune diseases commonly have alterations in the BACH2 gene.
Genome-wide association studies, which analyze genetic variants among people to determine whether specific variants are associated with particular traits, showed that DNA from patients with diverse autoimmune disorders often had minor alterations in the BACH2 gene, which laid the foundation for this research. The team found that if mice lacked the Bach2 gene, their cells became inflammatory and the mice died of autoimmune diseases within the first few months of life. When they re-inserted Bach2 (using gene therapy) into Bach2-deficient cells, the ability to produce regulatory cells was restored. These findings have implications for treating cancer as well as allergic and autoimmune diseases. The scientists are now working toward manipulating the activity of the Bach2 gene, with the goal of developing a new cancer immunotherapy. (NIH authors: R. Roychoudhuri, K. Hirahara, K. Mousavi, J.J. O’Shea, N.P. Restifo, D. Clever, C.A. Klebanoff, Z. Yu, M. Rao, P. Muranski, J.G. Crompton, L. Gattinoni, M. Bonelli, G. Sciumè, G. Vahedi, H. Takahashi, Y. Kanno, H. Zare, V. Sartorelli, B. Dema, J. Rivera, H. Liu, D. Bedognetti, E. Wang, F.M. Marincola, G. Punkosdy, and V. Hoffmann; Nature 498:506-510, 2013)
NIH TO REDUCE THE USE OF CHIMPANZEES IN RESEARCH

NIH
Pumpkin, a 24-year-old chimpanzee at the Alamogordo Primate Facility (APF), Alamogordo, N.M., loves coconuts and kiddie swimming pools. APF is a chimpanzee reserve where no research is conducted.
NIH announced recently that it plans to substantially reduce the use of chimpanzees in NIH-funded biomedical research and to designate for retirement most of the chimpanzees it currently owns or supports. NIH Director Francis Collins accepted most of the recommendations made by an independent advisory council (http://dpcpsi.nih.gov/council/working_group.aspx#Summary) for implementing a set of principles and criteria (http://iom.edu/Activities/Research/Chimpanzees.aspx) defined by the Institute of Medicine (IOM). NIH plans to retire more than 300 government-owned chimpanzees and to retain, but not breed, up to 50 chimpanzees for future biomedical research. The chimpanzees that will remain available for research will be selected based on research projects that meet the IOM’s principles and criteria for NIH funding. The chimpanzees designated for retirement could eventually join more than 150 other chimpanzees already in the Federal Sanctuary System, which is overseen by NIH.
NIH’s decision “culminates more than two years of intensive deliberations among NIH leadership, independent chimpanzee experts, researchers, bioethicists, and members of the public,” said James M. Anderson, NIH deputy director for program coordination, planning, and strategic initiatives, whose division oversees the NIH Chimpanzee Management Program. “We are grateful to all who have contributed their insight and expertise during the advisory process.”
NIH’s full response to the recommendations and public comments can be found at http://dpcpsi.nih.gov/council/working_group.aspx.
This page was last updated on Thursday, April 28, 2022
