Research Briefs
NHGRI, NIDDK, NCI, ORS: ELEVATED HORMONE FLAGS LIVER PROBLEMS IN MICE WITH METHYLMALONIC ACIDEMIA
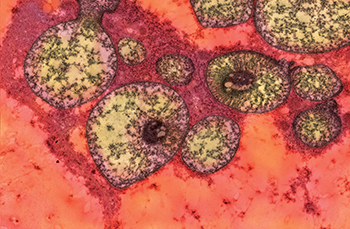
CREDIT: PATRICIA M. ZERFAS, ORS
NHGRI: The figure is an electron micrograph showing abnormally shaped and structured mitochondria in the liver of a mutant mouse that models methylmalonic acidemia.
NHGRI researchers led a team that discovered that a hormone, fibroblast growth factor 21 (FGF21), is extremely elevated in mice with liver disease that mimics the same condition in patients with methylmalonic acidemia (MMA). MMA is a genomic disease that impairs a person’s ability to break down food proteins and certain fatty acids. The condition affects roughly one in 50,000 children born in the United States and can be detected through newborn screening. Children with MMA suffer from frequent life-threatening metabolic crises when they encounter a minor viral illness or other stressors such as trauma, dietary imbalance, or surgery. They must adhere to a special low-protein diet and take various supplements their entire lives.
Based on the discovery, clinicians treating patients with MMA will be able to measure FGF21 levels to predict how severely patients’ livers are affected and when to refer patients for liver transplants. The findings also might shed light on more common disorders such as fatty liver disease, obesity and diabetes by uncovering similarities in how MMA and these disorders affect energy metabolism.
“We can use this information today to ensure that patients with MMA are treated before they develop severe complications,” said senior author Charles P. Venditti, who’s a senior investigator in the NHGRI Medical Genomics and Metabolic Genetics Branch.
The NHGRI team will next assess the role of FGF21 pathways in other symptoms seen in MMA. Their goals are to understand what defines the vulnerability to stress in MMA to better diagnose life-threatening metabolic crises that occur in patients, test new genomic therapies and find treatments that work for every patient. (NIH authors: I. Manoli, J.R. Sysol, M.W. Epping, L. Li, C. Wang, J.L. Sloan, A. Pass, J. Gagné, Y.P. Ktena, N.S. Trivedi, P.M. Zerfas, V. Hoffmann, M. Abu-Asab, M.G. Tsokos, D.E. Kleiner, A.G. Elkahloun, J. Schnermann, R.J. Chandler, and C.P. Venditti, JCI Insight 3:e124351, 2018; DOI:10.1172/jci.insight.124351)
NCI: A BACTERIA TOXIN TRIGGERS RUPTURE OF CANCER CELLS LEADING TO THEIR DEATH
Inflammation serves to kill invading bacteria and viruses. Certain molecules on the surface of the microbes can trigger an inflammatory cascade, and one example of such a molecule is lipopolysaccharide (LPS). Cells can react to LPS by triggering a process called pyroptosis that causes the cell to burst and die. The released cell contents attract blood and lymphatic cells that in turn kill the LPS-producing bacteria. This prevents the bacteria from multiplying and spreading.
LPS was used in the very early days of medicine to treat cancer, although it has fallen out of favor because it causes severe side effects, such as a hyperinflammatory response (sepsis) that can result in death. It was not known exactly how LPS kills cancer cells, which has limited its use.
NCI researchers have now shown that a protein called secretoglobin family 3A member 2 (SCGB3A2), which is produced by the cells that line the lung airways, binds to LPS. Tests on mouse immune cells and lung-cancer cells grown in the laboratory showed that the resulting SCGB3A2-LPS complex can then bind to a cell-surface protein called syndecan 1. This binding enables LPS to enter the cell and trigger pyroptosis and cell death.
To confirm the role of SCGB3A2 in pyroptosis, the researchers examined tumor growth in mice that were not able to produce SCGB3A2. These mice developed more tumors than normal mice, but tumor growth was suppressed when mice were injected with SCGB3A2.
Currently available cancer immunotherapy is targeted primarily to the host immune cells whereas the NCI study shows the possibility to directly target cancer cells. These findings could potentially lead to new types of cancer treatments and may provide clues for understanding the nature of many cancers that are refractory to cancer immunotherapy mediated by immune cells.
However, it remains to be examined whether molecules that trigger pyroptosis, like LPS, could also be used to treat cancers other than those from the lung. Further work is also needed to understand in more detail how SCGB3A2 and LPS work together to cause cancer-cell death. The combination of cancer immunotherapy and stimulation of cancer-cell self-destruction could greatly advance the treatment of cancer patients. (NIH authors: S. Yokoyama, Y. Cail, M. Murata, T. Tomita, M. Yoneda, L. Xu, R.E Cachau, and S. Kimura, eLife 7:e37854, 2018; DOI:10.7554/eLife.37854)
NIDA: MAINTAINING THE ER PROTEOME UNDER CALCIUM DEFICIENCY
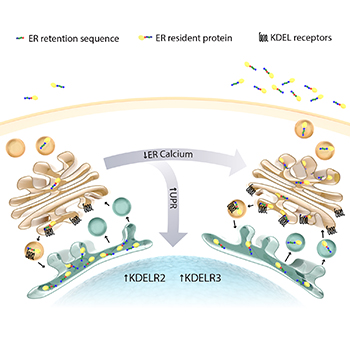
CREDIT: SUSANNE BACK
NIDA: This graphic shows an exodus of proteins from the endoplasmic reticulum (ER) in response to the loss of luminal ER calcium. The cell works to maintain the calcium-deprived ER proteome by upregulating KDEL receptors.
A new paper published by NIDA scientists describes, for the first time, a novel pathological mechanism that may contribute to a variety of disease states. Within the cells of our body, many of the proteins and lipids are produced by a structure within a cell called the endoplasmic reticulum (ER). The ER also serves as the cell’s primary reservoir of calcium. Each of the resident ER proteins is kept in the ER by a tail sequence called an ER-retention sequence. If a protein escapes the ER, its tail is recognized by a receptor called a “KDEL (Lys-Asp-Glu-Leu) receptor” and returned to the ER. This function is critical to the maintenance of a healthy body. The study shows that when the calcium concentration drops in the ER, the cell proteins overwhelm this retrieval mechanism and redistribute to the outside of the cell. As a result, protein functions are lost in the ER, and unwanted expression occurs outside the cell, which may contribute to a variety of disease pathologies related to the depletion of ER calcium such as diabetes, stroke, Alzheimer disease, cardiac diseases, and substance-use disorders. This greater understanding of the most delicate mechanisms in the body suggests that stabilizing ER calcium or increasing KDEL receptors has therapeutic potential. (NIH authors: K.A. Trychta, S. Back, M.J. Henderson, and B.K. Harvey, Cell Reports 25:1829–1840, 2018)
NIDDK, NHLBI, NIAMS: T-REG CELLS SUPPORT INTESTINAL WOUND HEALING
A subset of regulatory T cells, called CD161+ Treg cells, accelerated wound healing and may also reduce inflammation in the gut, according to scientists from NIH and the United Kingdom. The study described the biological mechanisms of CD161+ Treg cells in humans, finding them to be a distinct, stable, and highly suppressive population of regulatory T cells. CD161+ Treg cells were also associated with regions of lower inflammation in Crohn disease. These findings suggest that infusion of CD161+ Treg cells may be a valid therapy for inflammatory bowel disease. (NIH authors: Y.-C. Chen, D. Chauss, H.-W. Sun, H.-Y. Shih, C. Kemper, M. Pirooznia, and B. Afzali, Nat Immunol 19:1403–1414, 2018; DOI:10.1038/s41590-018-0230-z)
NIEHS: MALE MICE GROW OVARIES AFTER SINGLE GENE TWEAK
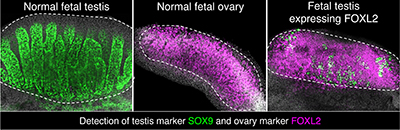
CREDIT: HUMPHREY YAO, NIEHS
NIEHS: The normal mouse testis, left, produces large amounts of a testis-specific marker called SOX9, shown in green. The normal mouse ovary, middle, produces large amounts of an ovary-specific marker called FOXL2, highlighted in pink. The genetically tweaked mouse testis, right, produces mostly FOXL2, but retains small amounts of SOX9.
By turning on the mammalian gene FOXL2, NIEHS scientists prompted male mice to grow ovaries. Among vertebrates, there is a wide variation in the signals that trigger sex determination of the gonads. In some types of turtles and alligators, sex is shaped by environmental cues such as temperature or the availability of nutrients. In humans, it is written in the composition of the sex chromosomes. Although there are differences, many of these organisms use the same proteins to guide sexual development, such as sex-determining region Y (SRY) and SRY-related box 9 (SOX9) in the testis and wingless-related integration site (WNT) family member 4 and forkhead box protein L2 (FOXL2) in the ovary.
Male mice were genetically engineered to produce FOXL2 protein in their testes. Their gonads morphed into ovaries, although they retained some molecular and structural features related to their original sex programming. Because much more is known about sex determination of the testis, the team wanted to better understand sex determination of the ovary.
The study suggests that sexual development is flexible and may be able to shift in response to different genetic or environmental factors. The findings could give insight into disorders of sexual development and defects in fertility. (NIH authors: B. Nicol, S.A. Grimm, A. Gruzdev, G.J. Scott, M.K. Ray, and H.H.C. Yao, Hum Mol Genet 27:4273–4287, 2018; DOI:10.1093/hmg/ddy312)
NIEHS: SOX17 PROTEIN IS CRITICAL FOR PREGNANCY
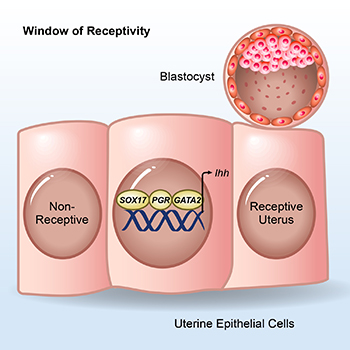
CREDIT: FRANCESCO DEMAYO, NIEHS
NIEHS: SOX17, the progesterone receptor (PGR), and GATA2, along with other transcription factors, bind near the Ihh gene to support embryo implantation.
Using mouse genomic data, genome editing, and bioinformatics, a team led by NIEHS scientists determined that the protein sex-determining region Y–related box 17 (SOX17) may be critical to a woman’s ability to become pregnant. The researchers found that the SOX17 transcription factor works with the progesterone receptor in female mice to regulate the ability of the uterus to support embryo implantation. The scientists hope they can use the information to eventually turn stem cells into healthy uterine-lining cells, which would help some women with infertility or uterine disease. SOX17 regulates both development of the uterus and the genes that support embryo implantation. Removing either the SOX17 binding site, which the team determined to be near the Ihh gene, or the entire SOX17 gene altered communication between uterine epithelial cells and the stroma. As a result, the uterus did not form properly and was unable to support pregnancy.
When SOX17 was present, it worked with the progesterone receptor to control the ability of the uterus to receive an embryo. The team found that mouse SOX17 used the protein Indian hedgehog homolog, which is coded for by the Ihh gene, to communicate with and regulate uterine genes. The finding has implications for treatment of infertility and diseases in which patients do not respond well to progesterone therapy—such as endometriosis, endometrial cancer, and some infertility cases. (NIH authors: X. Wang, T. Wang, S.-P. Wu, and F.J. DeMayo, Nature Comm 9:Article number 4421, 2018; DOI:10.1038/s41467-018-06652-w)
NIAID: EYES OF CJD PATIENTS SHOW EVIDENCE OF PRIONS
CREDIT: RYAN KISSINGER, NIAID
Ocular tissues tested by real–time quaking-induced conversion.
NIAID scientists and their colleagues at the University of California (UC) at San Diego and UC–San Francisco have found evidence of the infectious agent of sporadic Creutzfeldt-Jakob disease (CJD) in the eyes of deceased CJD patients. The finding suggests that the eye may be a source for early CJD diagnosis and raises questions about the safety of routine eye exams and corneal transplants. Sporadic CJD, a fatal neurodegenerative prion disease of humans, is untreatable and difficult to diagnose. About 40 percent of sporadic CJD patients develop eye problems that could lead to an eye exam, meaning the potential exists for the contamination of eye–exam equipment designed for repeat use. Further, cadaveric corneal transplants from undiagnosed CJD patients have led to two probable and three possible cases of disease transmission, the researchers say. Previous studies have shown that the eyes of CJD patients contain infectious prions, though the distribution of prions among the various components of the eye was not known.
To address this question, the scientists recruited 11 CJD patients who agreed to donate their eyes upon death. The researchers found evidence of prion infection throughout the eyes of all 11 deceased patients using real–time quaking-induced conversion (RT-QuIC), a highly sensitive test NIAID scientists developed that detects prion-seeding activity in a sample as evidence of infection. The RT-QuIC test is used in clinical settings to diagnose sporadic CJD in people. The researchers will continue their work to evaluate accessible eye components or fluids as feasible diagnostic–testing sources. They also plan to use other RT-QuIC tests to evaluate the eyes of patients with Alzheimer disease, Parkinson disease, and dementia with Lewy bodies to determine whether infectious proteins from those disease processes are present. (NIH authors: C.D. Orrù, B.R. Groveman, and B. Caughey, mBio 9:e02095, 2018; DOI:10.1128/mBio.02095-18)
NEI: NIH SCIENTISTS COMBINE TECHNOLOGIES TO VIEW THE RETINA IN UNPRECEDENTED DETAIL
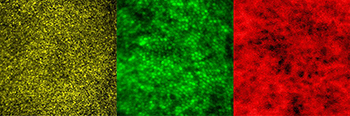
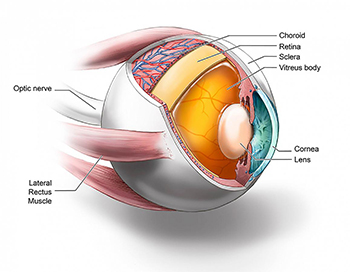
CREDIT: JOHNNY TAM, NEI
Images show multimodal technique that use adaptive optics and angiography to simultaneously see photoreceptors (left), retinal pigment epithelial cells (center), and choriocapillaris in the living human eye.
By combining two imaging modalities—adaptive optics and angiography—NEI investigators can see live neurons, epithelial cells, and blood vessels deep in the eye’s light-sensing retina. Resolving these tissues and cells in the outermost region of the retina in such unprecedented detail promises to transform the detection and treatment of diseases such as age-related macular degeneration (AMD), a leading cause of blindness among the elderly. Biopsied and postmortem tissues are commonly used to study disease at the cellular level, but they are less than ideal for watching subtle changes that occur as a disease progresses over time. Technologies for noninvasively imaging retinal tissues are hampered by distortions of light as it passes through the cornea, lens, and the gel-like vitreous in the center of the eye.
The NEI researchers combined adaptive optics with indocyanine green angiography, an imaging technique commonly used in eye clinics that uses an injectable dye and cameras to show vessel structures and the movement of fluid within those structures. In an observational study involving 23 healthy subjects, the researchers found that the multimodal approach enabled them to see for the first time a complex unit of cells and tissues that interact in the outermost region of the retina. The unit includes light-detecting photoreceptors; retinal pigment epithelial cells, which nourish the photoreceptors; and the surrounding choriocapillaris, a layer of capillaries that supply the outermost region of the retina with blood.
A range of diseases, including AMD, Alzheimer disease, and atherosclerosis, disrupt the outermost region of the retina. The ability to visualize live retinal cells and tissues may shed new light on these conditions and could help doctors identify early signs of disease before a person has symptoms, when the disease may be more likely to respond to treatment. (NIH authors: H.W. Jung, T. Liu, J. Liu, L.A. Huryn, and J. Tam, Commun Biol 1:article 189, 2018; DOI:10.1038/s42003-018-0190-8)
NCCIH: STUDY EXPLAINS BEHAVIORAL REACTION TO PAINFUL EXPERIENCES
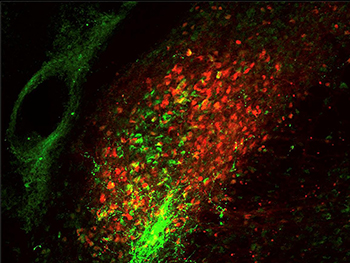
CREDIT: ARNAB BARIK, NCCIH
Two different groups of parabrachial neurons, one expressing calcitonin gene-related peptide (green) and the other expressing substance P (red).
Exposure to uncomfortable sensations elicits a wide range of appropriate and quick reactions, from reflexive withdrawal to more complex feelings and behaviors. To better understand the body’s innate response to harmful activity, researchers at NCCIH have identified activity in the brain that governs these reactions. Using heat as the source of discomfort, the researchers conducted experiments on mice that showed that bodily responses to pain are controlled by a neural pathway involving heightened activity in the spinal cord and two parts of the brainstem. The researchers set out to describe the brain pathway that controls motor responses and involuntary behaviors when the body is faced with painful experiences. Experiments showed that the parts of the brainstem involved in this circuit are the parabrachial nucleus (PBNI) and the dorsal reticular formation in the medulla (MdD). A specific group of nerve cells in the PBNI was activated when animal subjects stood on a hot surface, triggering escape responses through connections to the MdD. These PBNI cells express a gene called Tac1, which codes for substances called tachykinins that participate in many functions in the body and contribute to multiple disease processes. The MdD cells involved in this circuit also express Tac1.
A different group of cells in the PBNI participates in the aspects of the response to noxious heat that involve the forebrain. Knowledge of this brain activity provides new insight into how the body responds to harmful, painful stimuli. The mechanism described in this study can help researchers better understand how pain is encoded in the brain. Further investigation of this circuit may increase understanding of how responses to pain are coordinated with other biological needs, such as feeding and reproduction. These findings may also offer opportunities to understand how the body becomes dysregulated during chronic pain. (NIH authors: A. Barik, J.H. Thompson, M. Seltzer, N. Ghitani, and A.T. Chesler, Neuron 100:1-13, 2018; DOI:10.1016/j.neuron.2018.10.037)
This page was last updated on Tuesday, April 5, 2022
