Catalytic Research
NIH researchers and their collaborators are learning more about fetal affects in mothers who experience vaginal bleeding during the first trimester; brain plasticity; the structure of rixosome, a Swiss army knife-like protein complex; and a call to action on social media addiction.
NICHD: VAGINAL BLEEDING IN FIRST TRIMESTER MAY AFFECT FETAL LIVER, BRAIN GROWTH
The Fetal 3D Study, a multisite clinical study led by NICHD, performed 2,634 3D ultrasound examinations from 2015 to 2019. NICHD researchers and their collaborators investigated whether first-trimester vaginal bleeding affected fetal growth patterns and organ development. Of about 19% of the singleton pregnancies monitored, the women who self-reported bleeding during the first trimester received up to five 3D ultrasound scans between 15 to 40 weeks of gestation. The examinations assessed fetal body composition (arm, abdomen, thigh, adiposity) and organ volumes (cerebellum, lung, kidney, liver). Linear mixed models incorporating gestational age trajectories were adjusted for maternal age, race, ethnicity, pre-pregnancy body mass index, parity, and infant sex.
Compared to women without bleeding, fetuses of women who had one day or more of first trimester vaginal bleeding exhibited smaller abdominal areas (75.1–264.0 mm² reduction) between 30 and 40 weeks, smaller fractional thigh volume (1.1–4.0 cm³) and fat volume (0.4–2.4 cm³) from 30 to 40 weeks, but larger cerebellar volumes (0.8–1.6 cm³) after 35 weeks. Fetuses of women reporting only 1 day of vaginal bleeding showed reduced liver volume (2.6–4.8 cm³) between 26 and 35 weeks.
These findings suggest that even transient first trimester bleeding may coincide with fetal organ and adipose tissue development implications. Serial 3D ultrasound offers a valuable tool for identifying subtle alterations in fetal growth trajectories that may be linked to early gestational complications. (NIH authors: A. Jean-Louis, J.L. Gleason, Z. Chen, K. Wagner, D. He, J. Grewal, and K.L. Grantz, PMID: 40812363)
NIMH: REWIRING OUR THOUGHTS ON BRAIN PLASTICITY
For more than half a century, neuroscience textbooks have taught a dominant theory of brain plasticity: That when a body part is lost, the brain’s map of that part is erased and commandeered by neighboring areas—sort of a conservation of precious brain real estate. This idea, based on decades of cross-sectional studies, has shaped our understanding of how the brain adapts to injury.
Now, an international study published in Nature Neuroscience, conducted in part at the NIH, challenges this dogma, revealing a fundamentally different and more resilient picture of the brain’s somatosensory system. The research provides compelling evidence that the brain's map of an amputated limb remains remarkably stable, even years after the limb’s physical absence.
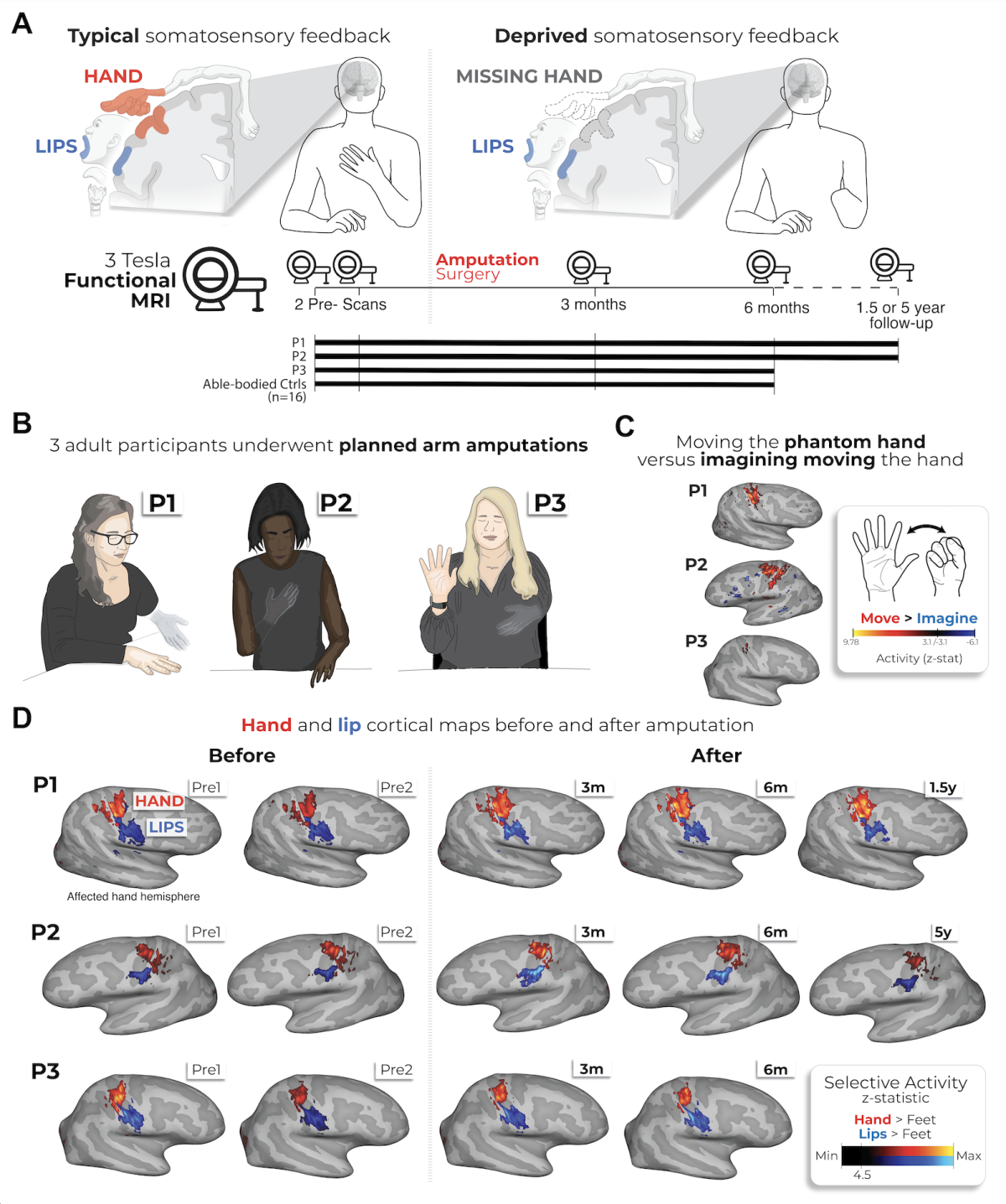
Instead of a traditional cross-sectional approach comparing a group of amputees with non- amputees, this study followed three adult participants who were scheduled to undergo planned arm amputations. The researchers — based mostly in the United Kingdom and at the NIH—conducted multiple functional MRI brain scans on each participant at different points: Twice before their amputation and then at 3 months, 6 months, and either 1.5 or 5 years post-amputation.
During these scans, participants were asked to perform movements of their fingers, lips, and toes. After the surgery, the participants continued to perform these same movements using their vivid phantom limbs. This unique before-and-after methodology allowed the scientists to directly track and compare the changes in the brain's cortical map over time within the same individuals.
Based on the findings, the research has provided conclusive evidence that the brain’s “fingerprint” of the missing limb is not erased but is, in fact, decently preserved. Movements of the phantom hand produced selective and stable activation in the brain’s primary sensorimotor regions, with neural patterns for individual phantom fingers remaining highly consistent with their preamputation state. There were no signs of remapping, either, such as by the lips and toes.
These findings have profound implications for future neurotechnologies and therapies. A stable neural representation of a missing limb provides a reliable and persistent blueprint for controlling advanced neuroprosthetics. The study also necessitates a reevaluation of the prevailing model for phantom limb pain, suggesting new therapeutic avenues that do not rely on the premise of cortical reorganization.
This research was co-led by Hunter Schone, who at the outset was a graduate student at University College London (UCL). Through the NIH Graduate Partnership Program (GPP), Schone tapped into the expertise of the NIMH Laboratory of Brain and Cognition, Section on Learning and Plasticity, led by Christopher Baker, a senior investigator. The GPP enabled Schone to work on his doctoral studies on both sides of the Atlantic, combining the amputation expertise at UCL with the neuroimaging expertise at NIH to produce a unique longitudinal study of amputation. Schone is now a postdoctoral associate at the University of Pittsburgh. (NIH authors: H.R. Schone, and C.I. Baker, PMID: 40841477)
CREDIT: NIMH
Study timeline and longitudinal brain maps showing preserved hand and lip activity
NIEHS: RIXOSOME REVEALED TO CONTAIN A STABLE CORE, FLEXIBLE ENZYMATIC MODULES
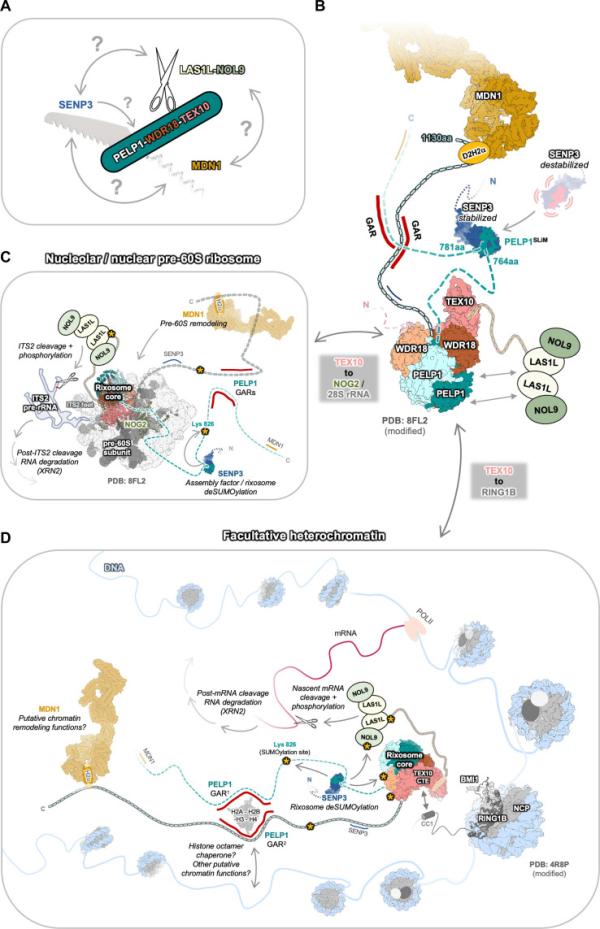
NIEHS researchers and their collaborators have mapped the architecture of the rixosome—a large “Swiss army knife-like” protein complex packed with molecular tools that regulate cellular functions.
The rixosome participates in RNA decay during processes such as assembly of the ribosome, a cellular structure that serves as the site of protein synthesis. It also plays an important role in maintaining heterochromatin, a tightly packed form of DNA characterized by its condensed structure. Yet the overall architecture of the rixosome has been hard to visualize because parts of it—called intrinsically disordered regions (IDRs)—lack a fixed 3D structure.
CREDIT: SCIENCE ADVANCES
Swiss army knife model of the rixosome.
Using structural and biochemical techniques, the researchers showed that a protein called PELP1 serves as the central scaffold of the rixosome. Enzymatic subunits assemble on a specific part of PELP1, an IDR that is rich in two types of amino acids called proline and glutamic acid. This flexible section helps PELP1 connect with another protein called SENP3. This protein is a SUMO-specific protease, which means it removes small proteins called SUMOs (small ubiquitin-like modifiers) that act like tags to control how a protein behaves, thereby regulating several key protein functions and gene activity.
Although SENP3 is associated with human diseases such as cancer, heart disease, and neurological disorders, it has not been clear how its protease activity is controlled. According to the authors, this research uncovers an activation mechanism for the SENP3 protease that could lay the groundwork for structural-based design of SENP3-specific protease inhibitors and regulators to treat cancer. (NIH authors: J. Gordon, A.M. Kaminski, S.R. Bommu, R.M. Petrovich, L.C. Pedersen, and R.E. Stanley, PMID: 40712028)
NIDA: A CALL TO ACTION ON SOCIAL MEDIA ADDICTION
Addiction experts published a recent call to action in the Journal of Addiction Medicine calling on experts to engage in rigorous longitudinal and clinical research to develop evidence-based prevention and treatment strategies to mitigate the adverse outcomes of compulsive social media use.
The authors note that problematic social media use exhibits behavioral parallels with substance use disorders, raising concerns about its potential classification within an addictive framework. Neuroimaging studies provide preliminary evidence of structural and functional brain alterations among heavy users that overlap with patterns observed in drug addiction, suggesting shared neurobiological pathways. Emerging data also indicate that excessive or maladaptive reliance on social media is associated with heightened symptoms of depression and anxiety, particularly in adolescents and young adults. These mood disturbances may, in turn, reinforce compulsive engagement, creating a self-perpetuating cycle of use and psychological distress.
Given the widespread prevalence of social media, the public health implications are considerable. Problematic use could exacerbate mental health burdens in vulnerable populations, underscoring the urgency of identifying risk factors and mechanisms underlying this behavior. More research is needed to clarify causality, delineate at-risk populations, and evaluate effective interventions. (NIH authors: R.D. Baler and N.D. Volkow, PMID: 40511818)
Note: The Journal of Addiction Medicine has a call for papers on studies funded through the NIH Helping to End Addiction Long-term (HEAL) initiative.
This page was last updated on Friday, September 5, 2025
