Too Much of a Good Thing
Yaron Rotman seeks treatments for an increasingly common cause of liver injury.

While many people might consider the brain and the heart to be the body’s most important organs, Dr. Yaron Rotman is firmly on team liver.
“The only reason we have bodies is to surround the liver,” he jokes. “My legs are meant to carry my liver from place to place.”
While Dr. Rotman is (probably) not entirely serious when he says that, there are many compelling reasons to be a fan of the liver. The multi-talented organ detoxifies our blood, helps us digest food, and even builds up stores of sugar and fat for later use as an energy source.
Unfortunately, the liver’s ability to store fat has crossed the line from helpful to harmful for the more than one in four Americans with non-alcoholic fatty liver disease (NAFLD), in which overloaded fat deposits in the liver threaten the organ’s health. Although the condition typically does not cause any noticeable symptoms in its initial stages, it can eventually progress to a concerning ailment called non-alcoholic steatohepatitis (NASH), in which the liver’s stored fat causes inflammation and impairs liver function. And while neither NAFLD nor NASH is an immediate threat like, say, liver cancer, that does not make them benign.
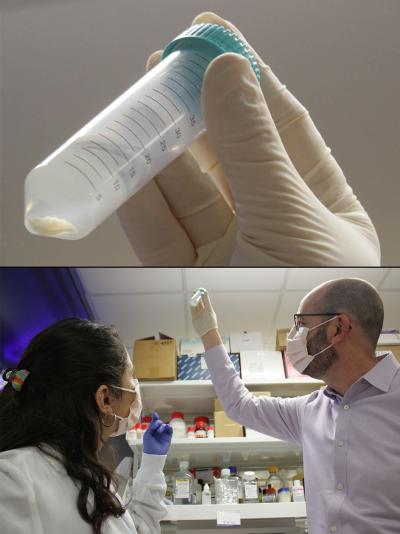
Dr. Rotman and postdoctoral fellow Lila González-Hódar examine a mouse's liver preserved in a test tube (top).
“Most people don’t have any symptoms, but symptoms and consequences are not the same thing,” Dr. Rotman explains. Just as we treat high blood pressure and cholesterol in otherwise healthy individuals to prevent future heart attacks and strokes, he argues, we need to short-circuit fatty liver disease before it causes more serious problems in the future. “NASH is a rapidly increasing reason why people are listed for liver transplantation. When someone is listed for a liver transplant now, that’s because of NASH that started 20 years ago.”
Of course, the liver can’t build up dangerously large stores of fat without calories from food; however, patients with NAFLD typically undergo clinical tests when they have not eaten in a while because what someone eats can affect blood test results, and undergoing more invasive procedures on a full stomach increases the risk of complications. Still, this approach leaves a lot out, and Dr. Rotman’s team hopes to fill in the gaps by investigating what happens in the liver after a meal.
“I believe that the liver injury seen in NASH is not a constant thing, but actually happens in bursts and may be worse after a meal,” he says. “I’m actually wondering if a single meal could be seen as a stress test for the liver.”
Indeed, Dr. Rotman’s research has demonstrated that after people with NAFLD drink a liquid meal, they burn less of the fat in it for energy over the next few hours compared to people without the condition, and their livers also create and release more of a class of fat molecules called diacylglycerols (DAGs). DAGs are associated with the formation of plaques in blood vessels, known as atherosclerosis, so his discovery might help explain why large stores of liver fat increase the risk for heart attacks and strokes.
“This may be a mechanism behind the acceleration of atherosclerosis in people with fatty liver disease that you wouldn’t know about if you only look at their blood during a fast,” Dr. Rotman says.

Dr. Rotman, research nurse specialist Elenita Rivera, and clinical fellow Harish Gopalakrishna demonstrate how they use a sealed space called a metabolic chamber for some of Dr. Rotman's studies. The chamber prevents air from freely moving between the inside and outside, allowing researchers to accurately measure how a person’s body processes food and burns calories. The experiments require inserting a tube called a cannula into the participant's nose (top-left) and hooking it up to a machine outside the chamber that measures and analyzes his or her breathing (top-right). The chamber was constructed with airlock-like features that allow medical staff to pass food to the participant (bottom-left) and draw blood from the participant's arm (bottom-right) without breaking the room's airtight seal.
Fortunately, fatty liver disease can be reversed through lifestyle changes like avoiding alcohol and losing weight, and scientists like Dr. Rotman are also searching for medications that could augment that approach. For instance, as part of an ongoing clinical trial, his team is treating NAFLD patients with a diabetes medication called semaglutide. As part of the study, the participants will also undergo liver biopsies before and after a standardized meal in the hopes that analyzing the liver samples will help reveal why semaglutide helps some people with fatty liver disease but not others.
“It’s likely that most medications for fatty liver disease will be needed for life, or at least long-term, so we want to detect futility early,” Dr. Rotman says. “Trying to figure out why some people respond to a medication and some do not, and using that to develop tools to predict the response to the medication before treatment or early on after treatment starts, will improve therapy.”
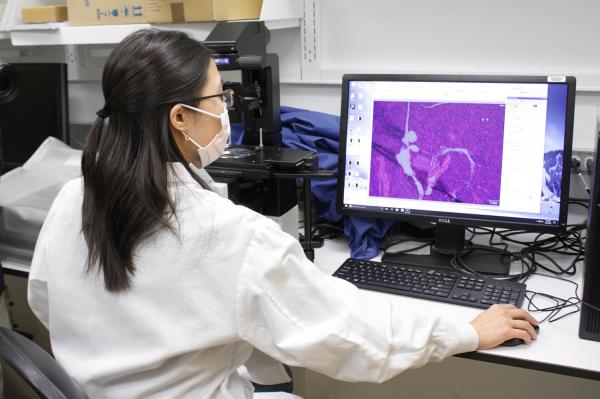
Postdoctoral fellow Allison Wing uses a microscope to get a close-up look at tissue from a mouse’s pancreas, an organ that can be damaged by NAFLD.
Dr. Rotman’s lab is also aiding the development of new treatments for fatty liver disease by examining how genetic differences influence the risk of developing NAFLD and the odds that the ailment will worsen into NASH. Several years ago, Dr. Rotman’s group was among the first to publish findings that linked variation in the HSD17B13 gene to differences in the likelihood of developing NASH. His group also showed that the gene produces an enzyme that helps the liver process fat, and mutations in the gene that disable the enzyme are protective against NASH.
Because the HSD17B13 gene is active in very few bodily tissues outside the liver, treatments for NASH that target the gene or the enzyme the gene produces are likely to have milder side effects than other potential approaches. It’s not surprising, then, that several pharmaceutical companies are now working on such therapies, and Dr. Rotman’s lab is assisting those efforts by investigating how the enzyme works in more detail.
“If inactivating that enzyme is good for us, why do we have it?” he wonders. “It must be good for us in another context. And even if the enzyme itself is found not to be an ideal target, understanding the pathways by which its inactivation leads to protection may point to other targets that are more suitable for intervention.”

Dr. Rotman with his research team. From left to right: Dr. Rotman, postdoctoral fellow Lila González-Hódar, postdoctoral researcher Wenqi Cui, and postdoctoral fellow Allison Wing. The inflatable shark hanging from the ceiling is the lab’s mascot, Lady DAG. DAG is a fat molecule that the lab is studying as part of its research on NAFLD, and “dag” means “fish” in Hebrew, the main language spoken in Israel, where Dr. Rotman was born and grew up.
As a physician-scientist simultaneously conducting clinical research while pursuing ‘basic science’ studies scrutinizing how the liver works, Dr. Rotman exemplifies the ‘bench-to-bedside’ ethos of the NIH Intramural Research Program. With his lab just a few steps away from the clinics where he meets with patients, it’s impossible for him to forget that his discoveries could lead to real improvements in people’s lives.
“I think that bridge between clinical and basic is unique to the NIH and allows us to do studies that may not be possible elsewhere,” he says. “I feel lucky and privileged to be able to work here and do what I do, and to be able to contribute to knowledge and help people.”
“This is the Mecca or the Vatican or the Temple Mount of science,” he adds. “There’s just no place like it.”
Yaron Rotman, M.D., M.Sc., is a Senior Investigator and head of the Liver and Metabolism Section in the Liver Diseases Branch at the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
This page was last updated on Wednesday, July 24, 2024