Shining a Light on Emotion
Mario Penzo uses cutting-edge genetic techniques to observe and influence brain circuits involved in stress and anxiety.

Dr. Penzo reviews experimental results with Yan Leng, his staff scientist and lab manager.
Human memory can be downright strange. People constantly forget where they left their keys, yet most can remember every single detail of the moment they scored a game-winning goal, or had their first kiss, or learned of a loved one’s death. The sound of an ice cream truck’s jingle might conjure images of care-free childhood summers, while the crack of fireworks could trigger overwhelming flashbacks to time spent in a war zone.
Clearly, periods of heightened emotional intensity can cause the brain to permanently and vividly etch certain experiences into memory. Understanding how the intricate electrical and chemical events occurring between neurons give rise to this phenomenon will likely be critical to figuring out the causes of psychological conditions like depression and post-traumatic stress disorder (PTSD). Addressing this fundamental research question is at the core of the research conducted by Mario Penzo, Ph.D. Using a set of cutting-edge scientific tools, Dr. Penzo and his team are able not only to observe how neurons fire in real time while animals perform complex behaviors, but also to intentionally alter the way those neurons function and observe the effects on those same behaviors.
“We want to understand how brain circuits operate under normal conditions and what their role in behavior is because we believe that deviations from the normal functioning of these circuits is what gives rise to mental illnesses,” Dr. Penzo says. “These are questions that scientists have had for a long time. Fortunately, thanks to the remarkable improvements in technological approaches, we can now address these questions more effectively.”

Postdoctoral fellow Sofia Beas peers through a microscope at some of the genetically modified neurons used in her experiments in Dr. Penzo’s lab.
Dr. Penzo’s research relies heavily on two techniques in particular. The first is optogenetics, in which neurons in an animal’s brain are genetically modified so that they become either more or less active when a particular wavelength of light hits them. Unlike a metal electrode that electrically stimulates all the neurons it touches, the cellular alterations that underlie optogenetics can be confined both to a specific area of the brain and to a specific type of neuron within that region, allowing researchers to study the specific contributions of those cells to cognitive processes.
“Optogenetics allows us to control the activity of specific neurons with precise timing,” Dr. Penzo explains. “If neurons are, for example, hyperactive and causing an animal to be in an anxious state, you can ameliorate those behaviors by reducing activity in that group of cells.”
Whereas optogenetics causes neurons to behave differently when they are exposed to light, another technique called calcium imaging induces genetic changes to neurons that cause them to emit light when they become active. This works by causing the cells to produce molecules that light up when they bind to the calcium ions that flow into firing neurons. Tiny microscopes embedded into the brains of animals can then measure the light given off when those neurons fire, even in freely moving animals. Moreover, just like optogenetics, the genetic changes that enable calcium imaging can be targeted to specific locations and cell types in the brain.

Dr. Penzo’s and Dr. Buonanno’s labs are both located in NIH Building 35A, the John Edward Porter Neuroscience Research Center. Having many of the IRP’s neuroscientists under one roof facilitates collaborations like theirs.
“These technical advancements have represented a major revolution in neuroscience research because they have enabled us to probe whether activity within certain brain circuits is causally related to specific behaviors,” Dr. Penzo says.
With the help of these two tools, Dr. Penzo has been able to glean important insights into how our brains produce, and are affected by, our emotions. For example, in a study published in 2018, Dr. Penzo teamed up with fellow IRP senior investigator Andres Buonanno, Ph.D., to explore why exposure to stress can make subsequent negative events even more impactful in both animals and humans. The study focused specifically on a brain region called the paraventricular nucleus of the thalamus (PVT), which was already known to be activated by stress. Dr. Penzo’s and Dr. Buonanno’s labs ultimately discovered that stress raises levels of a chemical called dopamine in the PVT and that this leads to cellular changes that cause neurons within the PVT to respond more strongly to subsequent stressful events.
They also used calcium imaging to show that stress activates dopamine-releasing neurons in another brain area called the locus coeruleus. The researchers used optogenetics to silence those locus coeruleus neurons in mice undergoing a stressful experience and found that doing so prevented the PVT from becoming hypersensitive to stress afterwards. The findings could help explain the origins of conditions like anxiety disorders and PTSD, in which a single traumatic event causes a person to respond much more strongly to other experiences even years later.
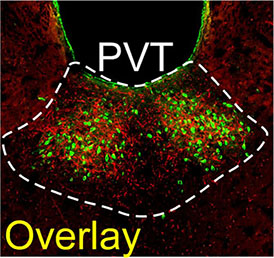
The PVT contains both dopamine-releasing neuronal projections from the locus coeruleus (red) and neurons that extend from the PVT to a brain region called the nucleus accumbens (green).
“One of the goals of our research is to determine the contributions of specific types of neurons to emotional processes, which helps us identify potential targets for pharmacological interventions that affect specific circuits,” Dr. Penzo explains. “We hope that these efforts can facilitate the development of future therapies for neuropsychiatric diseases.”
However, for Dr. Penzo, running a lab at the NIH isn’t just about generating new discoveries. It’s also important to him that he pass on his own knowledge by mentoring younger researchers. Growing up in the Dominican Republic, he lacked role models who could provide a glimpse of what life as a neuroscientist might be like, and as the head of a lab he continues to observe the struggles that occur when young people don’t know of many scientists who share their backgrounds. In one case, an African American postbaccalaureate trainee in his lab was accepted into a top neuroscience graduate program but was hesitant to accept the offer because, according to Dr. Penzo, “he felt there weren’t many people there who looked like him.”
“This sort of reminded me of some of my own reflections at that time in my own career,” Dr. Penzo says. “I feel fortunate that I was able to convince him that going to this place and getting himself a top-notch education will put him in a position to serve as a role model to others so that future generations don’t have to be concerned with this.”
“I think sometimes it can be challenging, when you don’t have any role models, to consider doing something,” he continues. “It’s one of the things that motivated me to do what I do.”
Mario Penzo, Ph.D., is the Chief of the Unit on the Neurobiology of Affective Memory at the National Institute of Mental Health (NIMH).
This page was last updated on Wednesday, May 24, 2023