Seeking Better Vision
Brian Brooks looks for ways to help families with childhood blindness.

Brian Brooks, M.D., Ph.D., and lab manager Prasad Alur, Ph.D. (right), develop treatments to improve vision in patients with inherited conditions
Like all physicians, Brian Brooks, M.D., Ph.D., wants to give his patients answers. He first realized the importance of answers when the parents of a child with coloboma (a congenital eye defect that can result in blindness) shared with him their joy at being pregnant again and asked if there was any risk of the unborn child having the same condition.
Brooks searched in vain, but the parents eventually left his office without an answer. The experience of being unable to provide solace in the face of uncertainty propelled Brooks toward a scientific career—one in which he could investigate inherited forms of childhood blindness.
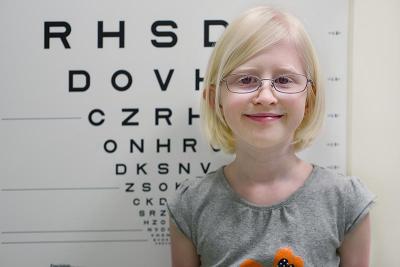
Lotus is a patient of Dr. Brooks’ with oculocutaneous albinism
Now, his clinic and lab focus on two diseases of the eye, using two different and complementary stages of scientific investigation: basic molecular research to understand the underpinnings of coloboma—a condition where the iris of one or both eyes has a distinct “keyhole” shape that can cause marked deterioration in sight; and development of novel therapeutics for oculocutaneous albinism—a disorder of melanin pigment in the skin, hair, and eyes.

Dr. Brooks examines Lotus’ eyes
“As a clinician investigator it’s important for me to be able to connect the work that I do in the clinic with my research in the lab,” Brooks says. “And I’m very fortunate to be able to do this type of work here, where the Clinical Center plays a central role in bringing bench and bedside together.”
In the last 10 years, Brooks has assessed more than 125 families, who receive full eye exams and blood tests at the NIH Clinical Center. By collecting clinical data from patients and immediate family members, they aim to improve diagnostics, genetic counseling, and understanding of the complex genetic underpinnings of both oculocutaneous albinism and coloboma.
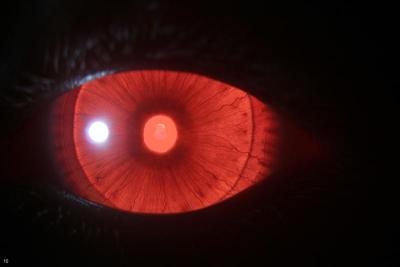
“Red eye” camera-flash reflex: Lotus’ eye shows iris transillumination due to reduced melanin pigment, rather than light passing only through the pupil as in fully-pigmented eyes
Brooks’ team has identified several new genes important in the critical stages of eye development and is working to determine the risk of developing coloboma associated with mutations in these genes. The congenital cause of coloboma occurs during the first trimester of pregnancy, making effective clinical intervention a challenge, but Brooks wants to improve genetic counseling, molecular diagnostics, and, potentially, prevention strategies for coloboma.

Lotus and her mother Kerry (left) visit Dr. Brooks’ clinic
The main reason for vision impairment in oculocutaneous albinism, however, develops after birth, so Brooks and his team investigate potential early therapeutic interventions in children. For reasons that remain unclear, this inherited form of albinism affects both skin pigmentation and retinal development, usually causing affected children to need some kind of visual aid.
Brooks hypothesized that, even without fully understanding the connection between pigment and retinal development, therapeutics might be developed that could alter pigmentation and also improve vision. In discussions with an IRP collaborator, William Gahl, M.D., Ph.D., Brooks learned of a compound called nitisinone (NTBC), which doctors use to treat children with a different metabolic condition, tyrosinemia type 1, which is due to the inability to break down the amino acid tyrosine.

Felix Onojafe, D.V.M., purifies DNA for analysis
“The first step in pigment production involves tyrosinase, which chemically modifies the amino acid tyrosine in the first and rate-limiting step of melanin production,” Brooks explains. “It turns out that this is the gene that is most commonly mutated in children with albinism. So, we had to ask, if we gave NTCB to a mouse with albinism could we stabilize the tyrosinase protein and improve the production of melanin?”
His group studied two animal models: the OCA1A albino mouse, which has no residual tyrosinase activity and the pink eyes and white skin (and fur) characterized by the full mutation; and the OCA1B mouse, also called a Himalayan mouse, which has low levels of tyrosinase activity and pale tan colored skin and fur.
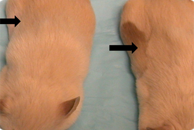
After shaving a patch of fur on two mice, the mouse receiving nitisinone grew darker replacement fur
The team found that when they shaved a small patch of fur on the Himalayan mouse and orally administered NTCB the fur grew back a dark brown color. In subsequent experiments they discovered that pregnant mice that received NTCB gave birth to pups with dark brown fur compared to the mother.
The proof of concept convinced Brooks to start a pilot clinical trial evaluating the effect of NTCB on adult patients with the OCA1B phenotype. Over 12 months, Brooks and his colleagues will measure changes to pigment in patients’ skin, hair, and eyes and evaluate whether vision improves.
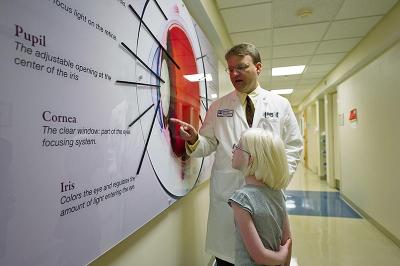
Dr. Brooks explains parts of the eye to Lotus
“Whether that is likely we can’t tell, because adults have a fully developed visual system, and we think the problem is more likely to be in development,” Brooks says. “However, by testing in adults first we will have a better idea of whether to pursue these types of studies in children.”
If the standard heel stick test identified newborn babies with oculocutaneous albinism, perhaps a short period of treatment could prevent the vision issues associated with the disease, which provides Brooks with hope that his research will lead to a brighter future for many children.
Brian Brooks, M.D., Ph.D, is a Principal Investigator in the Unit on Pediatric, Developmental and Genetic Ophthalmology at the National Eye Institute (NEI) and Adjunct Faculty at the National Human Genome Research Institute (NHGRI).
This page was last updated on Wednesday, May 24, 2023