No Assembly Required
Ken Yamada investigates the molecular basis of cell motility and adhesion.
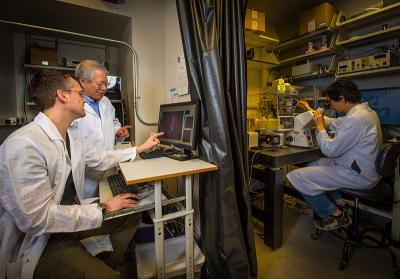
Staff Scientist Andrew Doyle and NIH Distinguished Investigator Ken Yamada discuss a 3D image of fibroblasts, while sabbatical researcher Hyun Koo examines migrating cells
A group of fluorescent green epithelial cells migrate rapidly and seemingly randomly within a forming organ in a video on a computer monitor. Eerily, the cells appear to have minds of their own as they bump and clump together, slowly forming an organized structure. Ken Yamada, M.D., Ph.D., explains that this transition from uncertainty to tissue organization is exactly what his group aims to figure out.
“These cells are naturally coming together and self-organizing to form a structure, in this case a duct,” Yamada says. “Understanding this phenomenon—how cells self-assemble to form a new entity, whether it’s a duct or a tumor—is key to understanding many crucial biological processes, from embryonic development to the development of disease.”

Postdoctoral fellow E. Emily Joo dissects mouse salivary glands
Yamada’s group investigates the mechanisms behind cell-matrix interactions – that is, how a cell or group of cells interacts with its environment. Understanding such interactions at the molecular level could influence how therapies are developed, including the distant goals of tissue remodeling and tissue engineering. Yamada can readily visualize that goal, having already collaborated on a patent for an artificial salivary gland. However, the dream of tissue engineering requires solid knowledge of how cells move and adhere to one another to form structures.
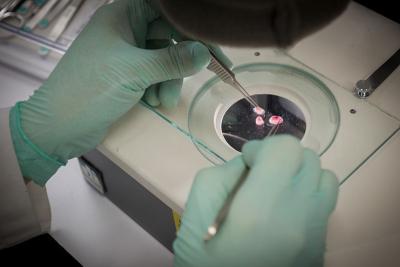
Embryonic mouse tissue provides cells for study
Over the years, a number of pathways, molecules, and mechanisms have been found to influence the process of cell motility. So, when Yamada and Ryan Petrie, Ph.D., recently published a paper in the Journal of Cell Biology suggesting an entirely new mechanism for cellular movement, they were also challenging the last 40 years of motility research.
“Traditionally, scientists have observed cells moving in two dimensions, either across plastic tissue-culture plates or upon layers of other cells,” Yamada explains. “In 2D, cells generally move by pushing out sheet-like structures called lamellipodia. However, when we visualized cells moving in physiological three-dimensional environments, we found a new state where the whole front of the cell advances, not as a lamellipod, but as an entirely new structure.”

Graduate students Jill Harunaga and Matthew Kutys (at the bench) conduct research with postdoctoral fellow Ryan Petrie supervising in the microscopy facility
Cell migration using the newly identified protrusions termed lobopodia contrasts with the current paradigm of cellular movement because it lacks the classic molecular signaling at the front of the cell, or leading edge—which Yamada and colleagues attribute solely to the 3D setting in which these cells are observed. The fibroblasts they study move through 3D extracellular matrix “scaffolds” that reflect a more natural environment for cellular motility than a flat 2D surface. This type of migration occurs without the polarized signaling molecules needed for migration in 2D environments.
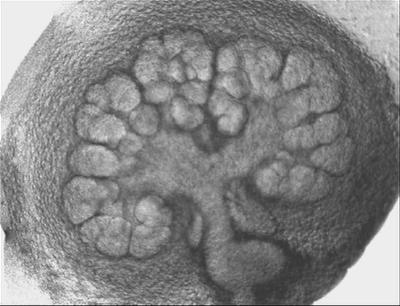
A brightfield microscopy image of a developing salivary gland
While Yamada focuses on defining the molecular basis of cell motility in three dimensions, individual areas of research within his lab are diverse, spanning a broad range of biology from embryogenesis to tumor metastasis. His lab follows a unique branch policy that allows post-doctoral researchers to take their individual research projects with them when they leave the lab. The policy, while ultimately career-establishing for many post-docs, raises challenges for the lab, but Yamada sees only positive outcomes.
“Allowing our post-docs to take their projects with them means we must avoid competing with them, essentially shifting our research path constantly,” Yamada says. “While some may view this as a disadvantage, I believe it’s an opportunity for us, because it challenges us to address questions in a novel way, perhaps by approaching them from a different angle.”

A confocal microscope image of a migrating human fibroblast in its 3D extracellular matrix
Original thought has been a hallmark of Yamada’s 30-plus-year career at the NIH. Starting as a physician-scientist, he soon realized his passion for benchwork outweighed his proclivity for patient duties. Relinquishing his clinical career to focus solely on laboratory research, Yamada then served in the National Cancer Institute before moving to the National Institute of Dental and Craniofacial Research, where he has continued to refine our understanding of cell-matrix interactions for more than two decades. Yamada describes working within the IRP as an opportunity to be unconventional in scientific thought and process. Drawing from an enormous network of potential collaborators, Yamada has found many opportunities to test new ideas and techniques, such as applying spinning-disk confocal microscopy to his work visualizing the three-dimensional movement of cells in vivo.

Dr. Yamada examining a confocal microscope image of a human fibroblast migrating in a collagen matrix
Success for Yamada has many definitions: seeing his post-doctoral researchers successfully transition to independent careers; mentoring new researchers on the importance of calculated risk; and pushing the boundaries of our scientific understanding on a daily basis. Looking at the clump of cells imaged on his computer screen, it’s a leap to imagine one day having tools available to encourage those cells to migrate, differentiate, and branch into a functional tissue or organ. But Yamada intends to make that goal our reality.
Kenneth M. Yamada, M.D., Ph.D., is an NIH Distinguished Investigator, Chief of the Laboratory of Cell and Developmental Biology, and Head of the Cell Biology Section of the National Institute of Dental and Craniofacial Research (NIDCR).
This page was last updated on Wednesday, May 24, 2023