Molecular Interventions
Christina Annunziata brings her unique expertise to bear on the problem of ovarian cancer.


“Inside the Beltway” is a phrase normally reserved for discussions of national politics, referring as it does to the highway that surrounds the Washington D.C. metropolitan area. Christina Annunziata, M.D., Ph.D., however, has developed her career inside the Beltway as a physician-scientist—first as a medical student and resident at Georgetown University, then rising through the ranks of training and career opportunities within the NIH Intramural Research Program (IRP) to become a tenure-track Investigator.
Over the years, Annunziata’s clinical responsibilities have involved her in many trials of investigational drugs with diverse targets, but her own research has remained focused on the NF-κB family of transcription factors. As a student, she studied NF-κB signaling in Hodgkin disease. As a Medical Oncology Fellow with Louis Staudt, M.D., Ph.D., she studied NF-κB in another blood cancer, multiple myeloma.
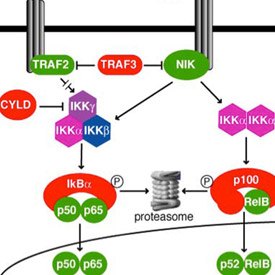
Now her own laboratory investigates the role of NF-κB signaling in ovarian cancer. “While working in the Staudt laboratory, I was also seeing patients with ovarian cancer as part of my clinical duties. I began to wonder whether NF-κB might play a role in this disease.”
Annunziata believes that understanding the nuances of NF-κB function in distinct cell types is key to its value as a therapeutic target. “NF-κB is a very interesting family of molecules. It consists of five subunits that form various combinations of dimers capable of coordinating the expression of multiple genes. NF-κB signaling is important for many different cell types. However, the pathway functions differently according to cell type.”

Annunziata and her team found that they could define a distinct gene expression signature that reflected increased NF-κB signaling in ovarian cancer cell lines and that disrupting this signature affected measures of cancer aggressiveness. The signature included genes associated with proliferation, survival, inflammation, adhesion, invasion, and angiogenesis—all the hallmarks of cancer. In collaboration with former IRP colleague Michael Birrer, M.D., Ph.D., now at the Dana Farber/Harvard Cancer Center, they found that this same NF-κB signature in primary tumors is associated with poor prognosis.
“There aren’t many direct NF-κB inhibitors available, mainly because of issues related to toxicity. So, we’re looking at other points in the pathway that might be more amenable to therapeutic intervention,” explained Annunziata. She is currently working with an investigational drug from Tetralogic Pharmaceuticals, TL-32711, which mimics the cell-signaling molecule, Smac. Under certain conditions, Smac shifts the balance of cell signaling from NF-κB -related proliferation to controlled cell death, exactly the shift that should stop cancer in its tracks.
Annunziata and her team are now studying the effects of Smac mimetics in ovarian cancer cell lines and also planning experiments in mouse models. If, as they predict, these drugs alter the NF-κB gene signature and associated cellular activity, the next step will be to move their work into human trials for recurrent ovarian cancer.

“Our hypothesis would be that patients with the highest levels of NF-κB activity would be most responsive to the drug,” said Annunziata who is acutely aware of the molecular heterogeneity of ovarian cancers. “It might be that only five percent of ovarian cancers will respond to a particular drug so, appropriate molecular testing is critical otherwise clinical trials will only ever see very low success rates,” she explained. They plan to hone in on the most responsive patient population by studying treatment response relative to the level of gene expression from an initial biopsy.
It is also often the case that a tumor evolves to evade a particular drug, which requires switching to another drug or another combination of drugs. “So, options are important,” concluded Annunziata, “which is one reason I continue to pursue my work in NF-κB. My personal goal, probably like many of my colleagues in the IRP, is to bring my research into the clinic.”
Christina Annunziata, M.D., Ph.D., is an Investigator in the Center for Cancer Research (CCR) at the National Cancer Institute (NCI).
Adapted from an article that appeared in CCR connections Volume 5, Issue 1
This page was last updated on Wednesday, May 24, 2023