A Reboot for Your Blood
Shahinaz Gadalla aims to improve outcomes for stem cell transplants.

By the time you finish reading this sentence, two people in the U.S. will find themselves in need of blood cells from another person. Unfortunately, blood transfusions are merely a stop-gap for patients who need blood not because of a surgery or injury but rather due to problems with the ‘hematopoeitic’ stem cells that produce blood cells in the first place.
Cancers like leukemia and other conditions prevent hematopoietic stem cells, located in bone marrow, from making enough new blood cells. The only cure is a hematopoietic stem cell transplant (HSCT), which involves destroying a patient’s own hematopoietic stem cells with radiation and chemotherapy before replacing them with cells from a healthy donor. However, regenerating a patient’s blood using someone else’s stem cells is a risky undertaking.
“Transplantation has definitely improved with time, but still a lot of patients die after this procedure,” says Dr. Shahinaz Gadalla, an IRP epidemiologist working to improve the outcomes for HSCT recipients. “We still have an average five-year survival after these procedures of 50 or 60 percent, so we still have a lot to do.”
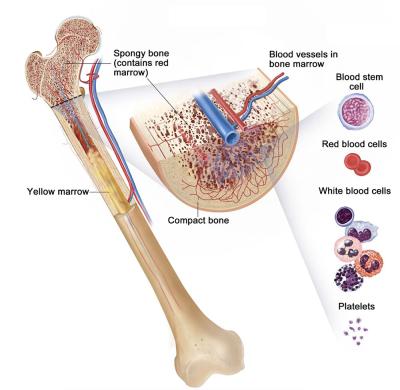
Hematopoeitic stem cells in bone marrow produce all three types of blood cells: red blood cells that carry oxygen, white blood cells that act as the foot soliders of the immune system, and platelets that help blood clot.
Beyond those dismal statistics, Dr. Gadalla also has a personal motivation for spending her days mining data about the procedure: her own daughter received an HSCT to treat a life-threatening medical condition. That experience brought home what it truly means for an HSCT recipient to have odds of survival similar to a coin flip.
“Even being in medicine, you don’t know all the ins and outs, but I saw it from the other direction,” Dr. Gadalla recalls.
One of the main reasons why HSCT is so risky is that the new immune cells produced by the donated stem cells often attack the recipient’s body, a condition called graft-versus-host disease that can be deadly if it gets out of control. To complicate matters, the drugs that doctors give transplant recipients to suppress their new immune systems and prevent graft-versus-host disease also make it easier for infections to spread unchecked inside the body.
Carefully selecting stem cell donors can lower the risk for graft-versus-host disease, which reduces the need for drugs that inhibit the immune system and leaves patients better able to fight off infections. Currently, donor selection depends mostly on matching donors and recipients based on genetic markers called human leukocyte antigens, as well as choosing younger donors in general. However, Dr. Gadalla’s research is showing that the biological age of a donor’s cells may be a more important factor to consider than his or her ‘chronological’ age in years.
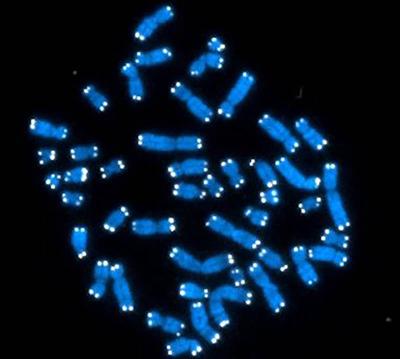
The 46 chromosomes that human DNA is organized into (blue) are capped with repetitive stretches of DNA called telomeres (white).
One way to gauge cellular aging is to examine telomeres, repetitive stretches of DNA located at the ends of chromosomes. Telomeres shorten each time a chromosome is duplicated during cell division, so older people typically have shorter telomeres because their cells have made many more copies of themselves. That being said, one 30-year-old may have much longer or shorter telomeres than another due to a myriad of genetic and environmental factors.
Intriguingly, research led by Dr. Gadalla suggests that patients who receive stem cell transplants from people with longer telomeres are more likely to still be alive five years afterwards. In fact, a donor’s telomere length may be a better predictor of a transplant’s outcome than their chronological age.
“In general, chronological age is kind of a washy marker,” Dr. Gadalla says. “It doesn’t tell you the full story.”
And telomeres may not “tell you the full story” either. In a study published in 2021, Dr. Gadalla’s research team and its collaborators found evidence that HSCT outcomes might be better when donors have a younger ‘epigenetic age,’ a measurement based on age-associated chemical tags at numerous locations on their DNA. That research examined data from more than 700 patients who received an HSCT from an unrelated donor to treat severe aplastic anemia, a condition that disrupts the body’s production of new blood cells. While most of the donors for those transplants had an epigenetic age close to their chronological age, some were what Dr. Gadalla calls “extreme agers,” with an epigenetic age 10 years or more greater than expected for their chronological age. Patients who received stem cell transplants from those donors were more than twice as likely to experience ‘chronic’ graft-versus-host disease after the transplant, a form of the condition that has a later onset and longer-lasting symptoms.

Dr. Gadalla discusses recent findings with the two members of her lab, postdoctoral cancer prevention fellow Kyra Mendez (right) and medical geneticist Maryam Rafati (middle).
“We think that this research will probably introduce a paradigm shift in donor selection," Dr. Gadalla says. “This can help get us away from the idea of chronological age and towards building a set of molecular markers that can define the cellular age of a donor.”
With that goal in mind, Dr. Gadalla’s team is now sifting through a massive batch of data from the Center for International Blood and Marrow Transplant Research (CIBMTR) on almost all HSCT procedures that occurred in the U.S. between 2000 and 2019. Using that treasure trove, which includes blood samples from donors and recipients, her research group hopes to link genetic variants, epigenetic age, and telomere length to the risk of dying after the procedure or developing post-transplant complications like graft-versus-host disease or the return of the illness that prompted the transplant in the first place.
“The beauty of this data is we can look for molecular signatures for better donor selection and build personalized risk calculators for recipients,” Dr. Gadalla explains. “We want to tailor treatment to the particular patient.”

Dr. Gadalla celebrates with her longtime mentor and collaborator, IRP senior investigator Sharon Savage, after Dr. Gadalla received the Research Highlights Award at the 2022 National Cancer Institute Scientific Investigators Retreat.
Dr. Gadalla is closer than ever to achieving that goal after more than 15 years at NIH and a steady ascent up the scientific hierarchy from a graduate student completing her Ph.D. as part of an IRP research group to running one of her own as a fully fledged senior investigator. The length of her stay at NIH is a reflection not only of her own determination, but also the unique scientific environment there — one that allows IRP investigators to do “high-risk, high reward research” and “raise questions that perhaps would be harder to tackle outside of NIH,” Dr. Gadalla says.
“I always wanted to stay at NIH because you kind of feel like your projects are your babies and you don’t want to leave them,” she continues. “I had visions for these projects, and I thought it made sense for me to build those visions where I started.”
Reaching the top of NIH’s scientific ladder, she adds, “comes with mixed feelings,” but those emotions only give her work more momentum.
“You feel accomplished because you feel that what you have tried to do was well-seen,” she says, “and you feel great responsibility to move what you started forward.”
Shahinaz Gadalla, Ph.D., M.B.Bch., is a senior investigator in the Clinical Genetics Branch at the National Cancer Institute Division of Cancer Epidemiology and Genetics (NCI DCEG).
This page was last updated on Tuesday, November 12, 2024