A Biological Betrayal
Mariana Kaplan aims to stop the immune system from harming the cells it is supposed to defend.

Dr. Mariana Kaplan (foreground) discusses experimental results with staff scientist Luz Blanco.
Any system functions best when it is carefully calibrated. A burglar alarm that blares when the front door is kicked in can be a life-saver, for example, but one that goes off every time the wind blows will soon have you pulling out your hair.
In the same way, an immune system that precisely targets disease-causing bacteria and viruses will help shield the body from illness, but one that sees our owns cells as a threat can cause life-altering consequences. It is the latter scenario that concerns Mariana Kaplan, M.D., as well as the more than seven percent of the United States’ population that suffers from an ‘autoimmune’ condition caused by an over-active immune system.
“In autoimmune disease, the immune system thinks that what is part of us is actually something that is foreign or external to us and starts attacking it,” Dr. Kaplan explains.
An immune system that cannot tell friend from foe can cause widespread harm that has serious effects on the quality and length of a person’s life, such as extensive damage to organs like the kidneys or lungs. It is also common for patients with autoimmune conditions to develop cardiovascular disease relatively early in life, which increases their risk for heart attacks and strokes. That’s not to mention the many more mundane symptoms of these diseases, which include fatigue, frequent fevers, and skin rashes.

Graduate student Jorge Romo Tena (background) and postdoctoral fellow Gustaf Wigerblad (foreground) prepare samples in the lab for analysis.
While treatments exist that combat these symptoms by suppressing the immune system, they are a blunt instrument, causing significant side effects because they affect many aspects of the body’s defenses. One of the main problems with therapies that broadly restrain the immune system is an increased risk of getting seriously ill if a patient does encounter a bacteria or virus.
“We need to come up with more targeted tools so that we can, ideally, only get rid of the bad players in the immune system but not affect the good parts that are so important to fight infections and detect cancers,” Dr. Kaplan says.
Dr. Kaplan has studied a number of autoimmune diseases, from joint-destroying rheumatoid arthritis to artery-inflaming vasculitis, but most of her efforts have been focused on what she calls “the poster child” for autoimmune diseases: systemic lupus erythematosus (SLE), more commonly referred to as ‘lupus.’ More than 5 million people around the world are thought to have some form of lupus, a number small enough to classify it as a rare disease but big enough that there is a larger pool of patients to study than many other autoimmune diseases. This makes lupus an ideal starting point for learning about the underlying causes of autoimmune disease in general.
“When diseases are very rare, it can take you a very long time to have enough patients to do good studies,” Dr. Kaplan explains, “so it’s helpful to extrapolate from a more common disease to a less prevalent disease, at least at the beginning.”
In this video, Dr. Kaplan describes her motivation to study lupus and the role that white blood cells called neutrophils play in the disease.
Since she arrived at NIH in 2013, Dr. Kaplan’s team has made significant progress towards zeroing in on the specific parts of the immune system that go rogue in people with autoimmune diseases like lupus. Her research has helped identify the key role of the ‘innate’ immune system, the portion of the body’s defenses that we are born with, in contrast to the ‘adaptive’ immune system, which learns and evolves over time as the body encounters and responds to disease-causing invaders. For example, the innate immune system routinely produces molecules called type-1 interferons that help the body fend off viruses, but people with certain autoimmune diseases produce much more of them when there is no virus around, and their immune cells also respond more strongly to type-1 interferons than those of healthy individuals. The effects of this are akin to giving an energy drink to an already rambunctious child in a room full of fragile objects — the immune cells, all revved up with nothing to do, start taking their frustrations out on the body’s own cells. Dr. Kaplan has found that type-1 interferons contribute to blood vessel damage in patients with lupus, a discovery that could help prevent some of the disease’s most severe complications.
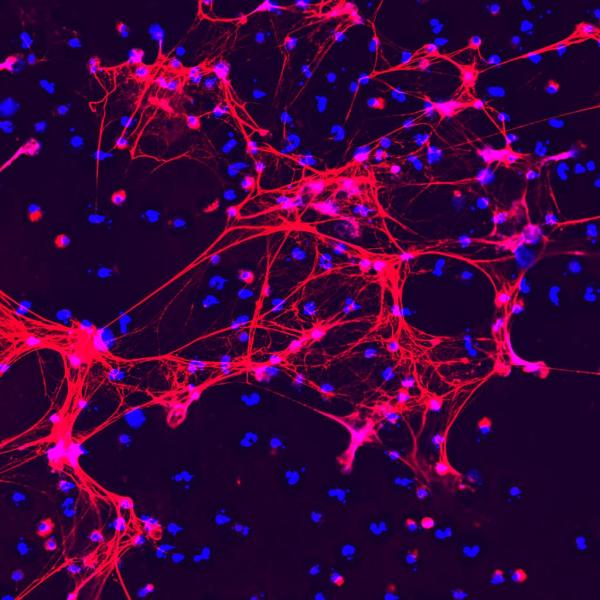
Immune cells called neutrophils produce sticky meshes made up of DNA (blue) and various proteins (red) in order to combat infections. Dr. Kaplan has discovered that this process goes haywire in people with autoimmune illnesses.
In addition, Dr. Kaplan’s group has discovered that a class of white blood cells called neutrophils, which are the innate immune system’s ‘first responders’ to an infection or injury, go haywire in many autoimmune diseases. In people with such illnesses, neutrophils over-produce anti-microbial webs called neutrophil extracellular traps (NETs). NETs play a key role in capturing and destroying microbes that have infiltrated the body. In autoimmune diseases like lupus, however, neutrophils will produce NETs even in the absence of a threat like an infectious bacterium, and these errant defensive weapons may encourage immune cells to attack the body’s own tissues and cause damaging inflammation. What’s more, Dr. Kaplan has found evidence that women have neutrophils that respond more strongly when exposed to type-1 interferons and produce more NETs than those of men, which may partly explain why autoimmune diseases are more common in women than in men.
“Type-1 interferons and neutrophils are both innate players of the immune system that are very important, and we think they act kind of in an alliance in patients with autoimmunity to promote even more damage or further contribute to abnormal immune function and blood vessel disease,” Dr. Kaplan says. “Ideally, we’d like to come up with one strategy that targets both, but also figure out a way to do that without making people more prone to infections. It’s a fine balance that we need to achieve, and a lot of our work is focused on finding that balance.”
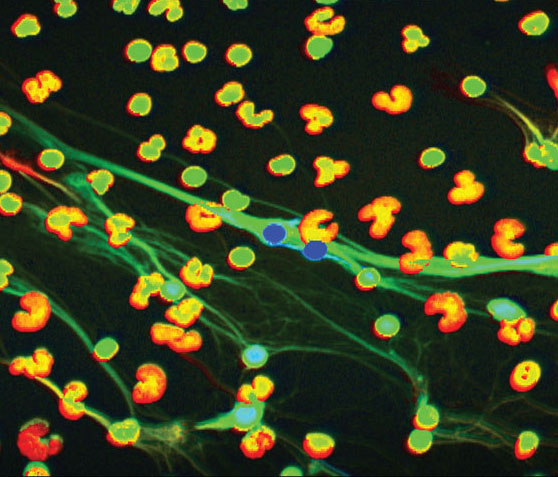
This image from a high-powered microscope shows low-density granulocytes (LDGs), a variety of neutrophil that Dr. Kaplan’s research has found to be a key player in autoimmune diseases.
One key to that is identifying the specific subsets of neutrophils that drive autoimmune disease. Scientists used to think that all neutrophils were the same, but Dr. Kaplan and others have shown that there are multiple varieties of neutrophils. Consequently, they aim to develop treatments that target only the disease-causing neutrophils while leaving the rest alone.
“Neutrophils are extremely difficult to study, and that, I think, has been a reason why they have been less understood and why the field of neutrophil biology has really lagged compared to what we know about other immune cells,” she explains. “New technologies have improved our ability to study these cells, and it has been an exciting time to get to better understand them.”
Access to those cutting-edge technologies is just one of the many ways the Intramural Research Program helps drive Dr. Kaplan’s research forward. Another is the diverse expertise of her colleagues across the IRP, who have encouraged Dr. Kaplan to take her work in new and exciting directions. For example, she is working with scientists at the National Institute of Dental and Craniofacial Research (NIDCR) to study Sjogren’s syndrome, an autoimmune condition that affects the production of tears and saliva, as well as the role of NETs in other inflammation-related diseases. Her lab is also partnering with researchers at the National Heart, Lung, and Blood Institute (NHLBI) to study blood vessel damage in inflammatory illnesses.

Dr. Kaplan with members of her lab. From left to right: Christopher Oliveira, Jorge Tomo Tena, Aracely Romero, Dillon Claybaugh, Luz Blanco, Gustaf Wigerblad, Dr. Kaplan, Norio Hanata, Eduardo Patino, and Shuichiro Nakabo. Members of the lab not pictured: Carmelo Carmona-Rivera, Laura Lewandowski, Sneha Das, and Faith Simmonds.
Like many scientists, Dr. Kaplan is also applying her unique expertise to the study of COVID-19, with the assistance of colleagues from all across the IRP. Specifically, her lab is examining how the novel coronavirus and the vaccine against it affect people with autoimmune conditions, as well as what role neutrophils might play in long-COVID, the collection of persistent symptoms experienced by many COVID-19 patients.
“The pandemic has taken us to places we didn’t expect before,” she says. “It has made immunology a particularly relevant field. We hope that what we learn will be helpful not only for COVID-19 but will also be complementary to our work in autoimmunity.”
“This is a new era with new types of vaccines and new viruses,” Dr. Kaplan adds, “and I’m sure more is yet to come.”
Mariana Kaplan, M.D., is a Senior Investigator and Chief of the Systemic Autoimmunity Branch at the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS).
This page was last updated on Wednesday, May 24, 2023