Monoclonal antibody prevents malaria infection in African adults
One dose of an antibody drug safely protected healthy, non-pregnant adults from malaria infection during an intense six-month malaria season in Mali, Africa, a National Institutes of Health clinical trial has found. The antibody was up to 88.2% effective at preventing infection over a 24-week period, demonstrating for the first time that a monoclonal antibody can prevent malaria infection in an endemic region. These findings were published today in The New England Journal of Medicine and presented at the American Society of Tropical Medicine & Hygiene 2022 Annual Meeting in Seattle.
“We need to expand the arsenal of available interventions to prevent malaria infection and accelerate efforts to eliminate the disease,” said Anthony S. Fauci, M.D., director of the National Institute of Allergy and Infectious Diseases (NIAID), part of NIH. “These study results suggest that a monoclonal antibody could potentially complement other measures to protect travelers and vulnerable groups such as infants, children, and pregnant women from seasonal malaria and help eliminate malaria from defined geographical areas.”
NIAID sponsored and funded the trial, which was led by Peter D. Crompton, M.D., M.P.H., and Kassoum Kayentao, M.D., M.P.H., Ph.D. Dr. Crompton is chief of the Malaria Infection Biology and Immunity Section in the NIAID Laboratory of Immunogenetics, and Dr. Kayentao is a professor at the University of Sciences, Techniques and Technologies (USTTB) of Bamako, Mali.
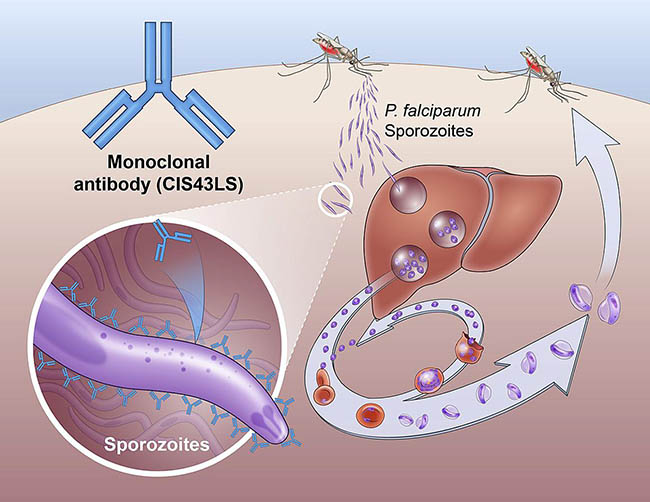
An antibody drug called CIS43LS prevents malaria infection by interrupting the lifecycle of the Plasmodium falciparum parasite. The antibody binds to and neutralizes sporozoites, the stage of the parasite transmitted from mosquitos to humans.
This page was last updated on Tuesday, November 1, 2022