Monoclonal antibody prevents malaria in U.S. adults, NIH trial shows
Larger trials enrolling infants and children are underway in Mali and Kenya
One injection of a candidate monoclonal antibody (mAb) known as L9LS was found to be safe and highly protective in U.S. adults exposed to the malaria parasite, according to results from a National Institutes of Health Phase 1 clinical trial published in The New England Journal of Medicine. Additional clinical trials evaluating if L9LS can prevent malaria over six to 12 months against seasonal and perennial transmission are underway in infants and children in Mali and Kenya, where malaria is endemic. The trial was sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of NIH.
“These early clinical trial results demonstrating that a monoclonal antibody administered subcutaneously can protect people from malaria are highly encouraging,” said NIAID Director Anthony S. Fauci, M.D. “A one-time intervention that protects against malaria for six months to a year could significantly reduce morbidity and mortality among children in malaria-endemic regions and offer an effective preventive tool for health care workers, military personnel and travelers to these areas.”
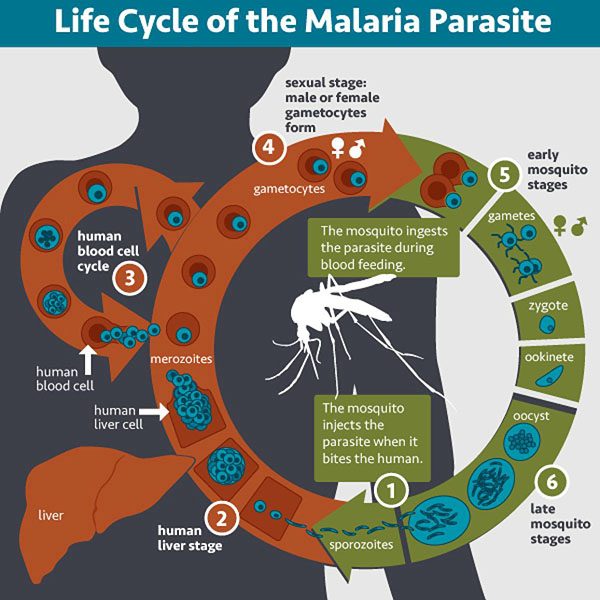
Malaria is a mosquito-borne disease caused by Plasmodium parasites. The World Health Organization estimates that in 2020, about 240 million people had malaria and about 627,000 of them died. A disproportionate burden of malarial disease is seen in Sub-Saharan Africa, where children under age 5 account for approximately 80% of all malaria deaths. A vaccine to prevent malaria is now available; however, its variable efficacy underscores the need for new interventions that offer high-level protection against disease.
This page was last updated on Tuesday, August 23, 2022