Preserving Cellular Health During Aging
Nathan Basisty looks for molecular markers to assess biological age and prevent future disease.

Our cells are constantly dividing. It’s how we grow from a single fertilized egg to a newborn baby with speciliazed organs and approximately 26 billions cells in all. As we age into adulthood, that number increases a thousand-fold.
But cells can’t divide forever. When a cell stops multiplying but has not died, it is said to be ‘senescent.’
“Senescence is a permanently stressed state from which a cell can never go back to normal,” says Nathan Basisty, a biochemist and principal investigator at the National Institue on Aging (NIA) interested in how senescent cells drive disease. “Senescent cells never start dividing again but will accumulate in virtually all of our tissues as we age, and they drive a lot of the illnesses that we associate with aging,” including Alzheimer’s disease and type 2 diabetes.
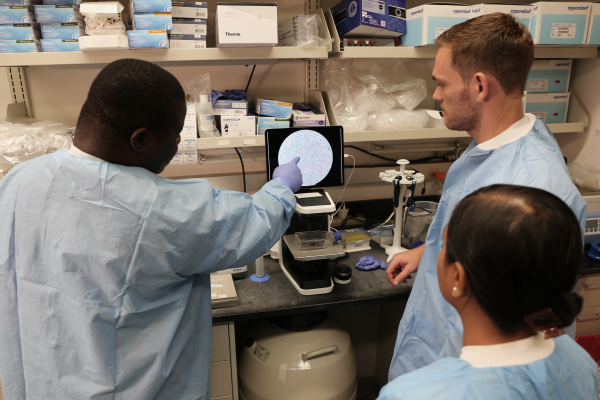
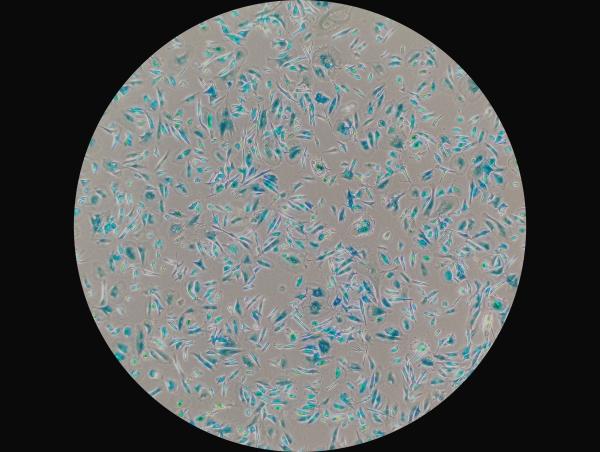
Visiting postdoctoral fellows Elikanah Adegoke (top left) and Reema Banarjee (top right) and Dr. Basisty examine senescent cells (bottom) dyed blue under a microscope.
Senescent cells secrete harmful molecules that trigger inflammation and disrupt the function of neighboring cells, similar to the way a rotten apple spoils the rest of the barrel. This flood of molecular pollutants can also spread to distant cells in other parts of the body by traveling through the bloodstream. Thousands of proteins, fat-based molecules known as lipids, and other, smaller compounds make up the infective mixture refered to as the senescence-associated secretory phenotype (SASP).
Dr. Basisty believes SASP could serve as a measure of a person’s biological age, which refers to the body’s physiological decline regardless of the number of birthdays celebrated. Because SASP-related compounds circulate in the blood, they could provide readily accessible indicators of a person’s ‘senescence burden,’ or the number of senescent cells they carry. As such, they could help gauge whether drugs that target those cells effectively lighten the load.
“If we have a molecular readout that tells us our biological age, then after giving a certain treatment, we could measure this molecular signature and tell whether or not this intervention is working,” says Dr. Basisty.
Those potential interventions include the use of ‘senolytic’ drugs that selectively kill senescent cells. Mice treated with senolytic drugs have shown a significant drop in senescent cells and, on average, have longer lifespans. However, more research into the effects of senolytic drugs is needed before they can be used on humans, especially considering that senescent cells aren’t all bad and eradicating them altogether could lead to other health complications. For example, one of the reasons we have senescence is as a defense against cancer. Cancer cells spread rapidly and uncontrollably, and senescence permanently stops proliferation. Additionally, the SASP has shown to promote healing after an injury, so ridding the body of all senescent cells could compromise processes that are necessary for a long and healthy life.
“We don't want to stop the process all together, but bringing these cells below a certain threshold is potentially therapeutic,” explains Dr. Basisty. “It’s a delicate balance.”
Before scientists can thread that needle, they must sift for it in the haystack that is the SASP. To cut through the clutter, Dr. Basisty’s team is using a technique called mass spectrometry that allows them to identify and quantify thousands of compounds in a single tissue sample. In a recent study, the group and their collaborators analyzed 1,301 proteins in 997 individuals between the ages of 21 and 102, ultimately identifying 651 proteins associated with age. Of those, 17 senescence-associated proteins were found to predict the risk of having two or more health chronic conditions, such as hypertension, heart disease, Alzheimer’s disease and other age-related conditions.
Dr. Basisty believes insights into the SASP could offer a wellspring for the future of personalized medicine. Not only could it indicate an individual’s senescence burden, but if refined enough, it could pinpoint which organs in that person are most affected.
“We want to know what proteins are uniquely secreted by a senescent liver cell versus a senescent brain cell,” he says.
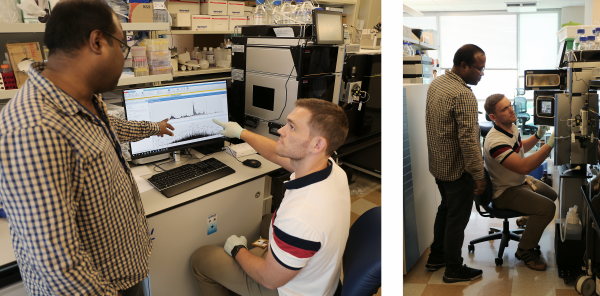
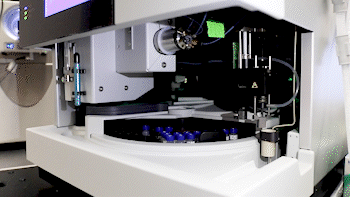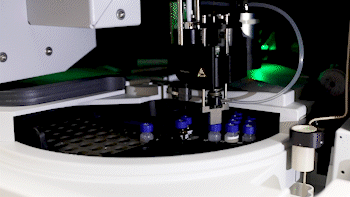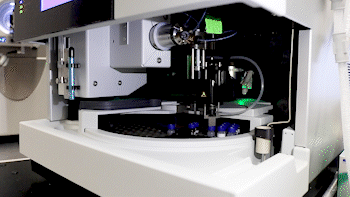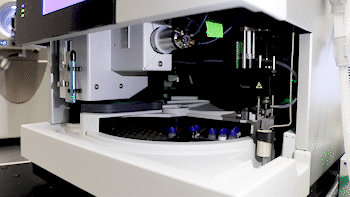
Dr. Basisty and biochemist Amit Dey (top) use mass spectrometry to analyze a sample (bottom) for compounds that could indicate ‘senescence burden.’
Nevertheless, free-floating proteins are only smoke signals of senescence. They can only tell scientists there’s a fire and potentially where its burning. To extinguish the source and develop therapeutics against senescent cells, Dr. Basisty is targeting proteins attached to the cells themselves. He is working to characterize the proteins exclusively found on the surface of senescent cells and exploit them as drug targets to selectively remove those cells while sparing their healthy neighbors.
Currently, Dr. Basisty’s group has its sights set on the surface proteins of senescent cells in fat tissue. Removing them could decrease the likelihood of developing metabolic disorders like diabetes.
“The hope would be that this mitigates the metabolic syndrome potentially caused by having senescence in fatty tissue and also hopefully mitigates aging overall,” explains Dr. Basisty.
Curbing the cellular effects of aging is an ambitious goal, one that requires validating findings from the lab to the clinc, which Dr. Basisty does by collaborating with other groups in the IRP and tapping into the many resources they share. This includes accessing the treasure trove of data collected as part of the Baltimore Longitudinal Study on Aging, which was initiated by NIA in 1958 and is the longest-running scientific study on human aging in the U.S.
“We have this unique opportunity to do studies on a scale that you don't really see outside NIH,” he says. “We can go to those studies and ask questions about senescence burden and which markers are changing with age in the blood and are they associated with lifespan, disease, or clinical outcomes.”

The Basisty lab. Top row left to right: Postbaccalaurete fellow Quinn Strassheim, biochemist Amit Dey, Dr. Basisty, visting fellow Elikanah Adegoke, and predoctoral fellow Bradley Olinger. Bottom row left to right: Postbaccalaurete fellow Delaney Rutherford, summer intern Ana Cunningham, postbaccalaureate fellow Thedoe Nyunt, visting fellow Mozhgan Boroumand, and visting fellow Reema Banarjee.
As Dr. Basisty and his collaborators investigate how and why time takes its toll differently on all of us, he emphasizes that the intent is not to live forever.
The Basisty lab. Top row left to right: Postbaccalaurete fellow Quinn Strassheim, biochemist Amit Dey, Dr. Basisty, visting fellow Elikanah Adegoke, and predoctoral fellow Bradley Olinger. Bottom row left to right: Postbaccalaurete fellow Delaney Rutherford, summer intern Ana Cunningham, postbaccalaureate fellow Thedoe Nyunt, visting fellow Mozhgan Boroumand, and visting fellow Reema Banarjee.
“It’s about reducing the period of frailty and loss of mobility at the end of life,” he says. “I would argue that nobody is against that. The main goal is to keep us healthy into old age.”
Nathan Basisty, Ph.D. is an NIH Distinguished Scholar and head of the Translational Geroproteomics Unit at the National Institute on Aging (NIA).
This page was last updated on Wednesday, July 24, 2024