One Gene, Three Disorders, and No Cures
Karen Usdin investigates how multiple copies of a short DNA repeat give rise to the Fragile X-related disorders.
Karen Usdin, Ph.D., leads research into the causes of a subset of the Repeat Expansion Disorders
With three billion base pairs, approximately 21,000 unique genes, and millions of repetitive elements of varying length and complexity, the human genome is a vast field of information from which to glean insights into human health and disease.
Principal Investigator Karen Usdin, Ph.D., is particularly interested in regions of the genome that contain many tandemly arranged copies of a short repeat unit—for example, the trinucleotide sequences CGG, GAA, or CTG. Long stretches of such repeats give rise to a variety of different human genetic disorders, known collectively as the Repeat Expansion Disorders. This group of human diseases includes Fragile X Syndrome (FXS), Friedreich ataxia (FRDA), Huntington disease, and Myotonic Dystrophy.

Postdoctoral fellow Dmitry Yudkin, Ph.D., preparing cells from a patient with FXS
Some repeats cause problems because they are located within the region of a gene that encodes a protein. These repeats alter the amino-acid sequence, and thus the properties, of the protein produced by that gene. However, other repeats, like the CGG-repeats responsible for FXS and the GAA-repeats responsible for FRDA, are located outside of the protein-coding region of the gene, making the relationship between the repeat and disease pathology less clear.
Postdoctoral fellow Xiao-Nan Zhao, Ph.D. (right), and predoctoral fellow Rachel Lokanga (left) discuss their genotyping data with Dr. Usdin
“Despite FXS being the most common heritable cause of intellectual disability, we still don't fully understand how the repeats, which are located in the FMR1 gene on the X chromosome, are responsible for disease symptoms,” Usdin says. “We do know that, when the number of repeats exceeds 200, the gene is silenced or switched off, but how this happens is still a mystery. Understanding the switch mechanism may have implications not only for FXS, but also for other diseases that result from inappropriate gene silencing.”
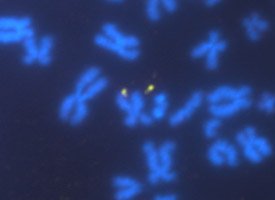
Chromosomes (blue) from a patient with FXS, showing breakage of the FMR1 gene (yellow) at the eponymous fragile site
Usdin’s team takes several different approaches to characterize the FMR1 gene switch and to see if it can be reset. To date, they have identified some key components of this switch and shown that, by inhibiting one of these components, they can flip the switch back on in patient cells in tissue culture. However, they have yet to determine what causes the switch to be turned off in the first place or whether it will ever be possible to switch the gene back on in patients.

Staff scientist Daman Kumari, Ph.D., examines neurons developed from induced pluripotent stem (iPS) cells generated from a patient with FXS
Her team is also interested in the fragile site that is associated with FX alleles since there is reason to think that this could be responsible for the high incidence of Turner syndrome (the loss of one X chromosome) seen in women with >200 repeats. Their data suggests that fragility may result from an intrinsic problem with replication of the CGG-repeats.
“We would also like to know how the repeat number gets to be so large in these patients,” Usdin says. “We know that the repeat number increases gradually at first, as the FMR1 gene is passed from parent to child and that large changes in repeat number only occurs when the gene is maternally transmitted. We have identified a number of key genes involved in this process but we still have a long way to go to fully understand this unusual type of mutation.”
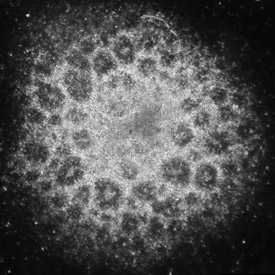
Neural rosette from Fragile X iPS cells
In addition, it has recently become apparent that even when someone inherits an FMR1 gene with <200 repeats, they can have problems. Surprisingly, these problems are completely different from those seen in people with >200 repeats. While people with >200 repeats have a learning disability and a variety of problems, including depression and anxiety, both men and women who inherit an FMR1 gene with 55–200 repeats are at risk of a neurodegenerative disease known as Fragile X-associated tremor and ataxia syndrome (FXTAS). Women are also at risk of a form of infertility known as Fragile X-associated primary ovarian insufficiency (FXPOI).
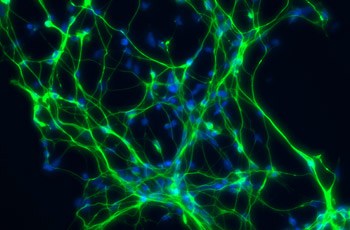
Neurons cultured from FXS iPS cells and stained with Map kinase (green) and DAPI (blue) provide insight into FX pathology
“Interestingly, while the FMR1 gene is switched off when the repeat number is greater than 200, the gene is actually overexpressed in people with 55-200 repeats,” Usdin says. “One theory for how these repeats cause FXTAS and FXPOI is that the RNA containing 55–200 repeats is somehow ‘toxic’, perhaps in a manner similar to the repeats in Myotonic Dystrophy, and that the overexpression of this RNA exacerbates disease pathology. Our demonstration that this RNA reduces the viability of cells, like neurons and oocytes, is consistent with this idea.”
Dr. Yudkin examines chromosomes from a patient with FXS using a fluorescent microscope
Currently, no cures exist for any of the Fragile X-related disorders. There are some drugs in clinical trials for the treatment of FXS, but finding treatments for FXTAS and FXPOI is complicated by the fact that researchers don’t yet understand how the repeats cause pathology. Since all three diseases result from epigenetic dysregulation, drugs that even partially reverse the abnormal gene expression might alleviate some of the symptoms.
A precedent for that approach can be seen with FRDA, which, like FXS, results from repeat-induced gene silencing. Work in mice done in other labs has demonstrated that it is possible for small molecules to increase expression of the affected gene, and clinical trials are underway to test such drugs in humans. In the meantime, Usdin’s lab continues working to understand how the repeats cause disease pathology in the Fragile X-related disorders, using a variety of approaches including the generation of induced pluripotent stem cells from patients and various disease-relevant cell types derived from these cells.

Dr. Usdin counts herself lucky to have been in close contact with many affected families over the years, both as a result of meetings at conferences, which in this field often include basic researchers, clinicians and affected families, and at lab visits she organizes for parents of affected children.
“Conversations with these families not only give us a better understanding of the impact of these disorders,” she says. “It also keeps us focused on trying to find better ways to help them.”
Karen Usdin, Ph.D., is a Principal Investigator for the Section on Gene Structure and Disease in the Laboratory of Cell and Molecular Biology at the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
This page was last updated on Wednesday, May 24, 2023