Forces of Nature
Keir Neuman applies physics to transform the field of biology.
Keir Neuman, Ph.D., develops new techniques for studying life
Surrounded by laser parts, lenses, and physics textbooks in his office, Keir Neuman, Ph.D., casually flips through a prestigious biomedical journal containing his team’s latest publication.
“It’s rather ironic really, because I’m a pure physicist with no formal training in biology,” Neuman says. “Yet, my colleagues here in the IRP believed in me and the value of this work in what is very much an emerging field.”
Custom laser setups are integral to Neuman’s research
Today, requests from biomedical scientists all over the world stream into Neuman’s email inbox, each seeking to tap his expertise on single-molecule imaging and manipulation. He patiently describes single-molecule biology as the real-world investigation of the enzyme diagrams at the end of many biomedical papers.
“Diagram 7, you know, the one where the authors show a stylized enzyme interacting with another molecule?” Neuman asks. “Well, we can now visualize and manipulate that exact scenario—and that’s single-molecule biology.”
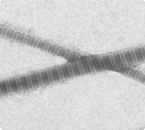
An electron-micrograph of two collagen fibers shows the characteristic bands spaced at 67 nm intervals (Photo: Sarah Hong)
The science behind Neuman’s work is all physics, which he applies to answer complex biological questions—often already considered answered long ago—that are now reexamined in the light of new findings from single-molecule biology.
A classic example of modified understanding concerns collagen and the enzymes that degrade it, known as collagenases. Collagen is a fibrous protein found in hair, skin, teeth, cartilage, and many other tissues in the body. It is fundamental to both normal biological processes, such as wound healing, and diseases, such as atherosclerosis. Collagen is also notoriously difficult to study biochemically due to its insolubility in water. To avoid studying the long, fibrous, insoluble tendrils of natural collagen, past biochemical research has only considered the monomer, a single subunit of this incredibly complex protein.
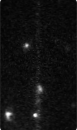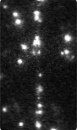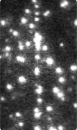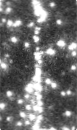
Time-series fluorescence micrograph of collagenase molecules attaching to a collagen fiber (linear formation) (Image: Susanta Sarkar)
Neuman and his colleagues took a different approach to learning about collagen’s role in biological processes, by first labeling the collagenase enzyme with a bright dye and visualizing a single molecule of the enzyme as it moves along a length of the collagen fiber in real time. It turns out that the collagenase enzyme moves in both directions along the collagen fibril, eventually attaching at “hot spots” where it cleaves the collagen in a unidirectional manner, a finding that completely redefined our understanding of collagen degradation.
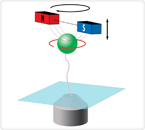
By rotating magnets (red, blue), Neuman’s team rotates a magnetic bead (green) attached to a DNA molecule (squiggly line), thereby twisting or “supercoiling” the DNA to study its behavior
Collagenase being anathema to many biochemists, Neuman wonders if his very naivety in the world of biology may actually help him pursue similar questions. The physicist-turned-biologist sees possibility beyond what has already been discovered. His vision has already found a new experimental paradigm, which in turn led to a whole new field of discovery and the subsequent deluge of collaboration requests from around the world.
Single-molecule biology has a second important element: manipulation at a tiny scale. Neuman and his colleagues have embraced technology referred to as “molecular tweezers.” Small glass, plastic, or magnetic spheres that serve as “handles” can be attached to biological molecules, organelles, or even entire bacterial cells, which scientists can manipulate using either a focused light beam or a magnet, essentially reproducing the famous Star Trek “tractor beam” to move an object.

Dr. Neuman and graduate student Tamara Litwin adjust an imaging lens
The biology resulting from this feat of physics can be quite breathtaking and include real-time physical analysis of molecular motors, RNA polymerases, swimming bacteria, and sperm, to name just a few examples. For the first time, Neuman and others in the field can characterize the forces biological molecules and organisms use to carry out their functions, including some in the range of pico-Newtons—about one-trillionth of the force required to stop a small apple from falling.
Unlike on Star Trek, the research applications in Neuman’s lab are not science fiction. The team often collaborates with Yves Pommier, M.D., and colleagues from the Center for Cancer Research (CCR) at the National Cancer Institute (NCI) to test novel chemotherapy agents using single-molecule biology techniques.

The team (left to right): Alice Sun,Ryan Harrison, Tamara Litwin, Marie-Paule Strub, Keir Neuman, Yeonee Seol, Junghoon In, Susanta Sarkar, Ambika Bumb
“There is a whole class of enzymes whose job it is to twist and turn our DNA into the complex coiled structures that are found within the nucleus of our cells,” Neuman explains. “By attaching a small magnetic particle to the DNA, we can twist or “supercoil” the DNA. Measuring how the DNA is uncoiled by enzymes termed topoisomerases, we can test the effects of a whole range of clinically important topoisomerase inhibitors.”
Their approach is unconventional, but it makes perfect sense to a physicist who appreciates the importance of looking beyond incremental scientific progress.
“After all,” Neuman says, “Edison didn’t invent the light bulb by making a better candle.”
Keir Neuman, Ph.D., is a Principal Investigator in the Laboratory of Single Molecule Biophysics at the National Heart Lung and Blood Institute (NHLBI).
This page was last updated on Wednesday, May 24, 2023