Decoding Telomere Biology and Cancer Risk
Sharon Savage leads a team to unravel the secrets of our telomeres.

Dr. Sharon Savage with Nancy, a participant in an NIH Clinical Center study of dyskeratosis congenita, prior to a bone marrow aspiration and biopsy procedure.
Chromosomes have little in common with either shoelaces or bombs, but telomeres—the repeated stretches of DNA that cap chromosome ends—are often compared to components of both.
As plastic tips (aglets) protect shoelaces from fraying, and bomb fuses burn down to imminent danger, telomeres maintain chromosomal stability and protect our genetic data—and when they wear away from aging or environmental stresses, DNA becomes vulnerable to degradation. Each time a cell divides, a process associated with normal aging, its telomeres get shorter, until the cell either undergoes apoptosis (programmed cell death) or senescence (the cell remains alive, but inactive).

Team meetings fuel a variety of research projects with new ideas.
Sharon Savage, M.D., has spent her career studying the association between telomere length and cancer risk, while searching for genetic markers that predict telomere biology disorders. Much of her work focuses on dyskeratosis congenita (DC), a rare and complex inherited cancer predisposition syndrome.
“Although extremely rare,” Dr. Savage explains, “because of its association with cancer and its established link to telomere biology genes, a better understanding of DC could have far-reaching implications for understanding cancer risk and etiology more broadly.”
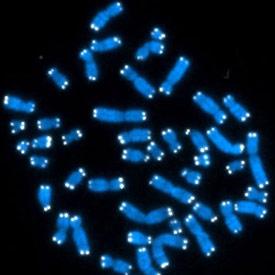
Human cells contain 46 chromosomes (blue), the ends of which are capped with protective telomeres (white) that vary in length.
When Savage joined the IRP’s Clinical Genetics Branch, mutations in three genes—DKC1, TERT, and TERC—accounted for approximately 40% of DC cases. The remaining 60% of DC patients still lacked an identifiable genetic source of the condition. Working as part of the Inherited Bone Marrow Syndromes (IBMFS) Cohort Study, at NIH’s National Cancer Institute, Savage’s team has since identified four additional genes with mutations associated with telomere maintenance that can cause DC.
Variation in a gene called TINF2 was their first major discovery. In collaboration with researchers at Stanford University, the team next showed that recessive WRAP53 mutations can cause DC—the first study to show that the abnormal location of telomerase, the protein that synthesizes DNA repeats at the ends of telomeres, in the cell is associated with DC. Most recently, her team discovered mutations in a DNA helicase and telomere maintenance gene called RTEL1 in several families, including two of Ashkenazi Jewish ancestry, a clinically severe variant of DC known as Hoyeraal-Hreidarsson syndrome (HH).

Dermatologists Dominique Pichard (left) and Edward Cowen (center) examine Nancy’s son Charlie’s skin and fingernails, two areas of the body frequently affected by DC and other telomere biology disorders.
“From here we collaborated with the Center for Jewish Genetics to define the frequency of RTEL1 mutations in this population and showed that they are common enough to be added to the prenatal testing panel for this population,” Savage says. “This story exemplifies why studying rare diseases is so important. Our RTEL1 discoveries wouldn’t have happened under other circumstances, and the findings will now be applied to help Ashkenazi and other Orthodox Jewish populations manage potential risks associated with this serious disease.”
Treatment for DC is challenging, and current options are limited, so Savage and Suneet Agarwal, M.D., a pediatric oncologist at Dana-Farber Cancer Institute and Boston Children’s Hospital, have formed the Clinical Care Consortium of Telomere Associated Ailments (CCCTAA) to identify new treatments and improve outcomes. First on their list is the urgent need to develop strategies for enrolling greater numbers of DC patients in clinical trials.

Her office can be a good place to catch Dr. Savage for a chat about new ideas.
In parallel, Savage is working with DC Outreach, a support group for affected families that she helped launch, to develop the first clinical guidelines for managing the disease. She also helps DC Outreach in their collaboration with the Genetic Alliance to create a database of DC patients. Thanks to Savage and others, there are now at least 13 known DC genes—all of them related to telomere biology.

Dr. Savage has devoted her career to thinking about ways to help people with telomere biology disorders.
“This work wouldn’t be possible anywhere else,” Savage explains. “First of all, large family studies like ours are important because they provide insight into more common cancers, but they require cross-discipline research collaborations. Second, because of the varied clinical manifestations of DC, our patients need access to a wide range of specialists. The Intramural Research Program combines these unique elements, allowing us to conduct fast-paced, high-risk projects with access to an unparalleled breadth and depth of expertise.”
Such attributes enabled Dr. Savage and her team to create a translational clinical genetics program devoted entirely toward the complex “defusing” of quickly shortening telomeres. Every day, and every patient who visits the clinic, brings them closer to identifying ways of slowing or interrupting that process.
Sharon Savage, M.D., is a Senior Investigator and Chief of the Clinical Genetics Branch in the Division of Cancer Epidemiology and Genetics (DCEG) at the NIH National Cancer Institute (NCI).
This page was last updated on Wednesday, May 24, 2023