And the Award for Best Supporting Cell Goes To…
Lisa Cunningham uses cellular stress to protect the inner ear from chemotherapy.

Dr. Cunningham leads an expert team working to understand the cellular and molecular responses to stress in the inner ear.
For many cancer patients, chemotherapy is a double-edged sword: possible tumor shrinkage with probable significant side effects. One of the irreversible effects of some chemotherapies is hearing loss, a devastating outcome for anyone that is especially problematic in children, as early hearing loss can lead to difficulties with speech development and learning.

Postdoctoral fellow Elyssa Monzack, Ph.D., uses a spinning disk confocal microscope to image living hair cells and supporting cells.
With a background that includes training in both clinical audiology and neuroscience, Lisa Cunningham, Ph.D., investigates the cellular biology of the inner ear for clues to preserving hearing in patients undergoing cancer treatment. Along the way, her team made a surprising discovery in that the answers they seek may reside, not in the sensory cells of the inner ear, which are called hair cells, but rather, in their neighboring supporting cells.
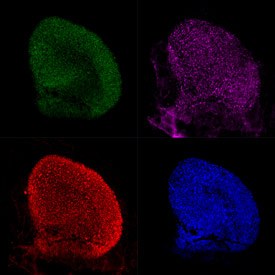
Mouse utricles with supporting cells expressing GFP (green) and labeled with anti-HSP70 (red). Hair cells are labeled with anti-myosin VIIa (pink), and all filamentous actin in the utricle is labeled with phalloidin (blue).
Cunningham and her team are examining what happens when auditory cells are exposed to “ototoxic” chemotherapy drugs, including cisplatin, one of the most successful anticancer drugs in clinical use. To do this, they utilize an in vitro preparation of whole-organ cultures of utricles from adult mice. The utricle is a balance organ in the inner ear that contains sensory hair cells and supporting cells that are similar to those in the hearing organ, the cochlea.

NIH-Oxford-Cambridge MSTP student Andrew Breglio dissects a mouse inner ear.
“Ototoxic drugs are a major stressor to hair cells, so we started our investigation with a family of stress-induced molecules called heat shock proteins (HSPs),” Cunningham explains. “These proteins have day jobs as molecular chaperones, helping other proteins get properly folded and localized within cells, but some members of the HSP family are rapidly induced by heat and a wide variety of other stresses. When they are induced by cellular stress, they can inhibit cell death. HSP induction is the most evolutionarily-conserved stress response in all of biology.”

Biologist Lindsey May infects supporting cells with an adenovirus to examine how the cells respond to stress.
Cunningham’s team showed that an increase in temperature from 37°C to 42°C for 30 minutes induced HSPs and reduced hair cell death in response to ototoxic drugs. One particular member of the HSP family, HSP70, had the most protective function of the group, but when the team went to investigate where HSP70 was being expressed in the tissue, they were surprised by the answer.
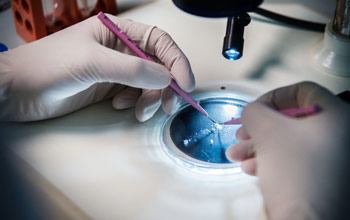
The utricle is a tiny balance organ in the inner ear, only about 0.5 mm in diameter, so handling it requires using precision tools viewed under a microscope.
“We thought this protective protein would be in the sensory hair cells, but it’s not—it’s in the supporting cells,” Cunningham says, “suggesting that the supporting cell is really playing the starring role here, protecting sensory hair cells from ototoxic damage.” Her team went on to demonstrate that the supporting cells secrete HSP70, which then promotes cell survival in hair cells.
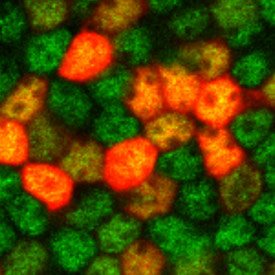
Supporting cells (labeled green with anti-Sox2) infected with adenovirus driving RFP expression (red).
Their basic finding was something Cunningham could start to translate to benefit people in the clinic. The question was how to induce HSPs in the inner ear. Using heat stress is clinically very challenging. The team has used pharmacological inducers of HSPs and shown that they are protective in animal models, but they can have significant unwanted side effects when administered systemically.
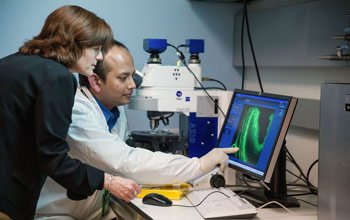
Dr. Cunningham (left) and visiting fellow Soumen Roy, Dr. rer. nat., examine hair cells of a mouse cochlea.
Scientists have known for about two decades how to pre-condition the inner ear using sound that is carefully calibrated to stress the inner ear enough to induce protective cellular mechanisms, but not enough to cause permanent hearing loss. So Cunningham set up an experiment to test the hypothesis that pre-conditioning noise—a type of ssssshhhhhh sound—would protect the hearing of animals that were then given the ototoxic drug cisplatin. When they tested different noise parameters, they found that a pre-conditioning exposure to noise protected the hair cells and hearing of the animals that received the ototoxic chemotherapy cisplatin.

“Our goal is to preserve or restore hearing in people receiving these lifesaving drugs,” Cunningham says.
“Our next step is to collaborate with our partners at the National Cancer Institute (NCI), who are already conducting clinical trials with cisplatin, to determine if exposure to pre-conditioned sound can inhibit cisplatin-induced hearing loss,” Cunningham says. “These patients have enough to contend with, so we’re moving as fast as we can to bring these insights from the cellular biology of stress into the clinic.”
Lisa Cunningham, Ph.D., is a Principal Investigator and Chief of the Section on Sensory Cell Biology at the National Institute on Deafness and Other Communication Disorders (NIDCD).
This page was last updated on Wednesday, May 24, 2023