NIH halts trial of COVID-19 convalescent plasma in emergency department patients with mild symptoms
Study shows the treatment is safe, but provides no significant benefit in this group
The National Institutes of Health has halted a clinical trial evaluating the safety and effectiveness of COVID-19 convalescent plasma in treating emergency department patients who developed mild to moderate symptoms of COVID-19, the disease caused by the coronavirus SARS-CoV-2.
An independent data and safety monitoring board (DSMB) met on Feb. 25, 2021 for the second planned interim analysis of the trial data and determined that while the convalescent plasma intervention caused no harm, it was unlikely to benefit this group of patients. After the meeting, the DSMB recommended that the National Heart, Lung, and Blood Institute (NHLBI), part of NIH, stop enrolling new patients into the study. NHLBI did so immediately.
Launched in August 2020, the Clinical Trial of COVID-19 Convalescent Plasma of Outpatients (C3PO) was being conducted at 47 hospital emergency departments across the United States and had enrolled 511 of the 900 participant recruitment goal. It was specifically looking at the effectiveness of COVID-19 convalescent plasma – blood plasma derived from patients who have recovered from COVID-19 – in adults who came to an emergency department with mild to moderate symptoms they had for a week or less. These patients also had at least one risk factor associated with severe COVID-19, such as obesity, hypertension, diabetes, heart disease, or chronic lung disease, but none were ill enough at the time to be hospitalized.
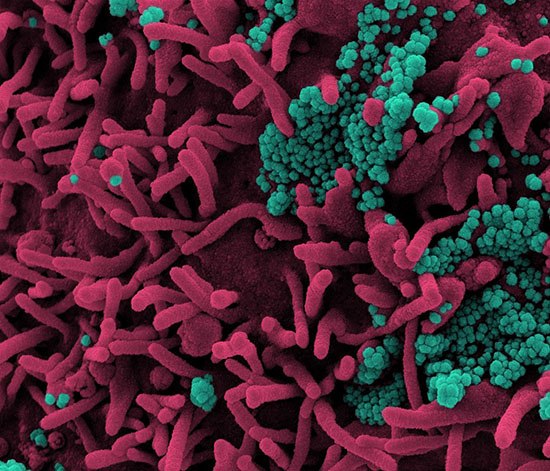
Colorized scanning electron micrograph of a cell (pink) infected with SARS-CoV-2 virus particles (teal), isolated from a patient sample.
This page was last updated on Friday, January 21, 2022