IRP, NIST researchers use artificial intelligence for quality control of stem cell-derived tissues
Technique key to scale up manufacturing of therapies from induced pluripotent stem cells
Researchers used artificial intelligence (AI) to evaluate stem cell-derived “patches” of retinal pigment epithelium (RPE) tissue for implanting into the eyes of patients with age-related macular degeneration (AMD), a leading cause of blindness.
The proof-of-principle study helps pave the way for AI-based quality control of therapeutic cells and tissues. The method was developed by researchers at the National Eye Institute (NEI) and the National Institute of Standards and Technology (NIST) and is described in a report appearing online today in the Journal of Clinical Investigation. NEI is part of the National Institutes of Health.
“This AI-based method of validating stem cell-derived tissues is a significant improvement over conventional assays, which are low-yield, expensive, and require a trained user,” said Kapil Bharti, Ph.D., a senior investigator in the NEI Ocular and Stem Cell Translational Research Section.
“Our approach will help scale up manufacturing and will speed delivery of tissues to the clinic,” added Bharti, who led the research along with Carl Simon Jr., Ph.D., and Peter Bajcsy, Ph.D., of NIST.
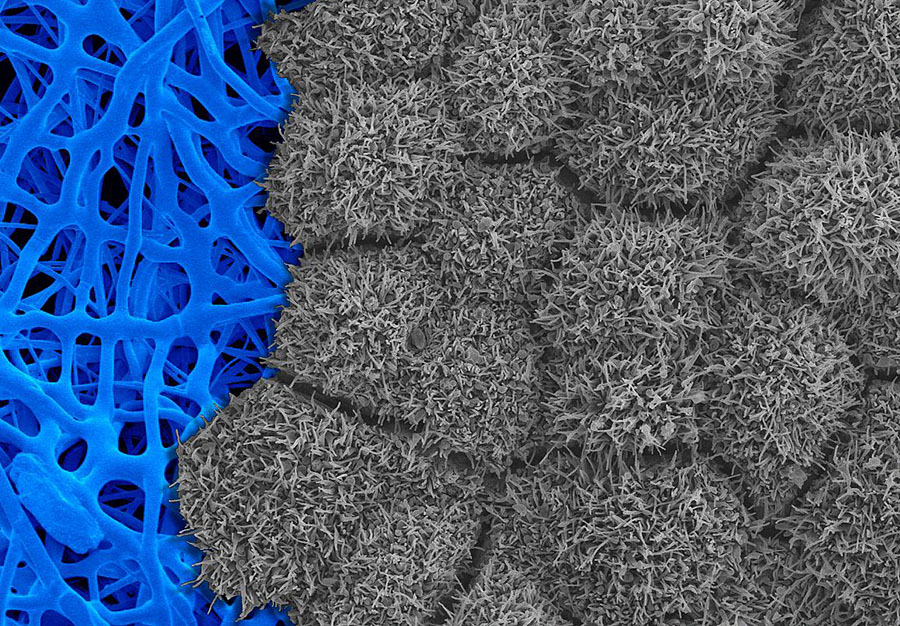
Scanning electron micrograph showing iPS cell-derived RPE tissue (gray) cultured on a fiber-based scaffold (blue).
This page was last updated on Friday, January 21, 2022