IRP begins first-in-human trial of a universal influenza vaccine candidate
Investigational vaccine designed to provide broad, durable protection from flu
The first clinical trial of an innovative universal influenza vaccine candidate is examining the vaccine’s safety and tolerability as well as its ability to induce an immune response in healthy volunteers. Scientists at the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, developed the experimental vaccine, known as H1ssF_3928.
H1ssF_3928 is designed to teach the body to make protective immune responses against diverse influenza subtypes by focusing the immune system on a portion of the virus that varies relatively little from strain to strain. The vaccine candidate was developed as part of a broader research agenda to create a so-called “universal” influenza vaccine that can provide long-lasting protection for all age groups from multiple influenza subtypes, including those that might cause a pandemic.
“Seasonal influenza is a perpetual public health challenge, and we continually face the possibility of an influenza pandemic resulting from the emergence and spread of novel influenza viruses,” said NIAID Director Anthony S. Fauci, M.D. “This Phase 1 clinical trial is a step forward in our efforts to develop a durable and broadly protective universal influenza vaccine.”
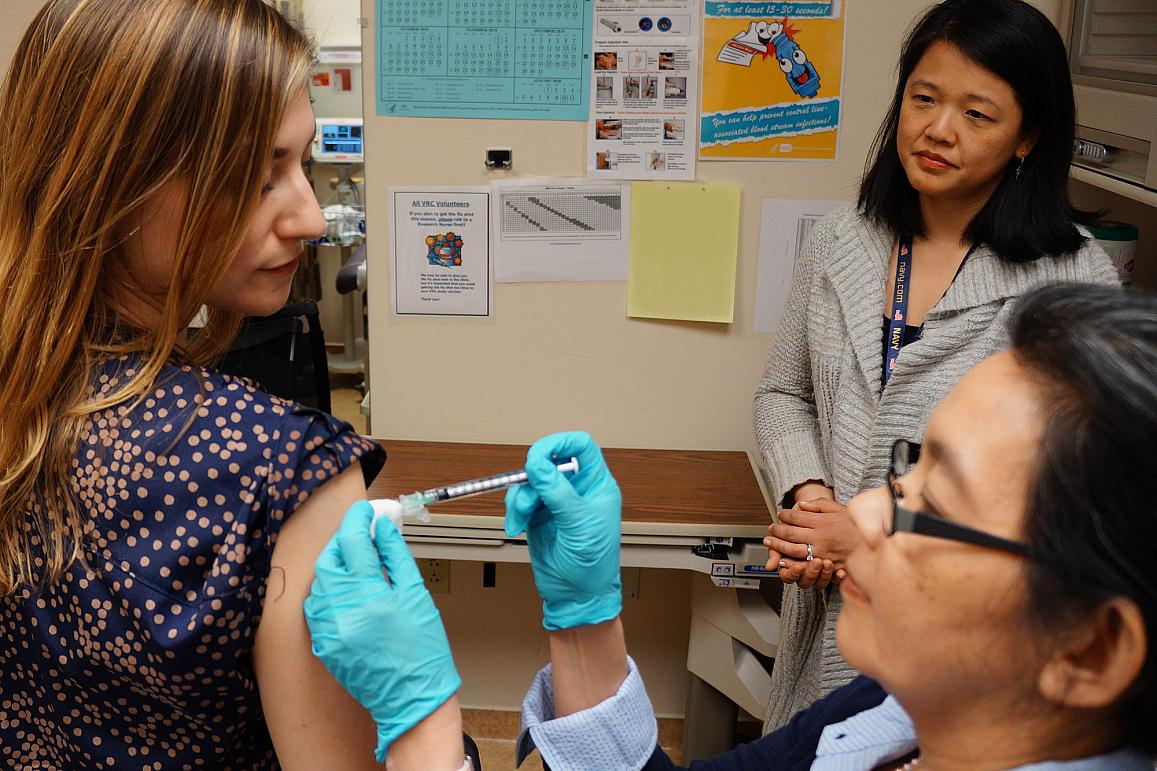
A healthy volunteer receives an experimental universal influenza vaccine known as H1ssF_3928 as part of a Phase 1 clinical trial at the NIH Clinical Center in Bethesda, Maryland. Scientists at NIAID’s Vaccine Research Center (VRC) developed the vaccine.
This page was last updated on Friday, January 21, 2022