Experimental NIH malaria monoclonal antibody protective in Malian children
Mid-stage trial shows treatment prevents infection, disease
One injected dose of an experimental malaria monoclonal antibody was 77% effective against malaria disease in children in Mali during the country’s six-month malaria season, according to the results of a mid-stage clinical trial. The trial assessed an investigational monoclonal antibody developed by scientists at the National Institutes of Health (NIH), and results appear in The New England Journal of Medicine.
“A long-acting monoclonal antibody delivered at a single health care visit that rapidly provides high-level protection against malaria in these vulnerable populations would fulfill an unmet public health need,” said Dr. Jeanne Marrazzo, director of the National Institute of Allergy and Infectious Diseases, part of NIH.
The clinical trial assessed two dose levels, with 19% of the 300mg-dose group and 28% of the 150mg-dose group developing symptomatic malaria, providing protective efficacy of 77% and 67% against symptomatic malaria, respectively. Among children who received placebo, 81% became infected with Plasmodium falciparum, and 59% had symptomatic malaria during the six-month study period. The authors note that the trial demonstrated for the first time that a single dose of a monoclonal antibody given by subcutaneous injection can provide high-level protection against malaria in children in an area of intense malaria transmission.
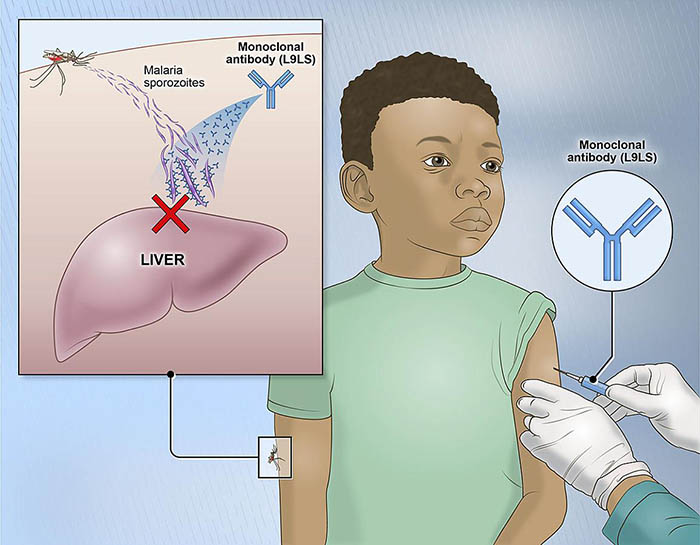
The experimental monoclonal antibody binds to and neutralizes 'sporozoites,' the form of the malaria parasite transmitted by mosquitoes that invades the liver to initiate infection.
This page was last updated on Friday, April 26, 2024