Experimental Ebola vaccines elicit year-long immune response
NIH reports final data from large clinical trial in West Africa.
Results from a large randomized, placebo-controlled clinical trial in Liberia show that two candidate Ebola vaccines pose no major safety concerns and can elicit immune responses by one month after initial vaccination that last for at least one year. The findings, published in the October 12 issue of the New England Journal of Medicine, are based on a study of 1,500 adults that began during the West Africa Ebola outbreak. The trial is being conducted by a U.S.-Liberia clinical research collaboration known as the Partnership for Research on Ebola Virus in Liberia (PREVAIL), established in 2014 in response to the request from the Liberian Minister of Health to the U.S. Secretary of Health and Human Services. The trial is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH) and involves scientists and clinicians from Liberia and the United States.
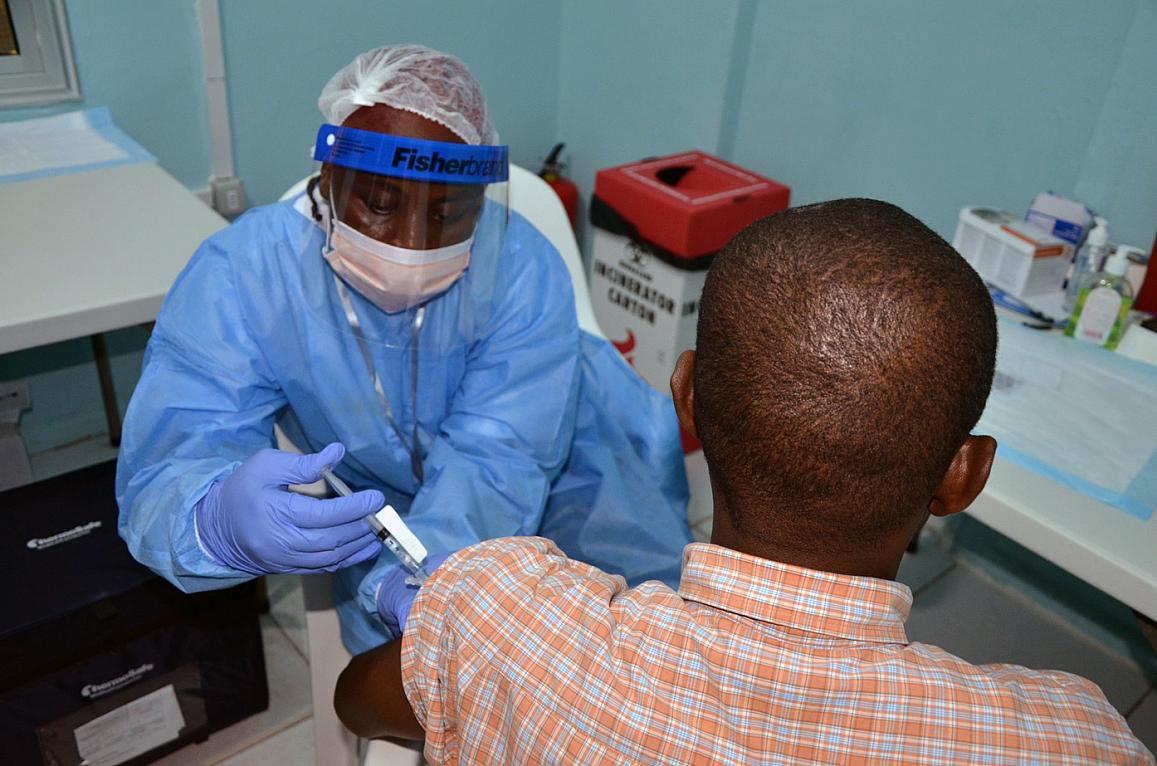
A volunteer receives an injection in the PREVAIL Ebola vaccine clinical trial in Liberia. Credit: PREVAIL
This page was last updated on Friday, January 21, 2022