Experimental antiviral for COVID-19 effective in hamster study
MK-4482 shows potential to prevent and treat SARS-CoV-2 infection
The experimental antiviral drug MK-4482 significantly decreased levels of virus and disease damage in the lungs of hamsters treated for SARS-CoV-2 infection, according to a new study from National Institutes of Health scientists. SARS-CoV-2 is the virus that causes COVID-19. MK-4482, delivered orally, is now in human clinical trials. Remdesivir, an antiviral drug already approved by the U.S. Food and Drug Administration for use against COVID-19, must be provided intravenously, making its use primarily limited to clinical settings.
In their study, published in the journal Nature Communications, the scientists found MK-4482 treatment effective when provided up to 12 hours before or 12 hours after infecting the hamsters with SARS-CoV-2. These data suggest that MK-4482 treatment potentially could mitigate high-risk exposures to SARS-CoV-2, and might be used to treat established SARS-CoV-2 infection alone or possibly in combination with other agents.
The same research group, located at Rocky Mountain Laboratories, part of NIH’s National Institute of Allergy and Infectious Diseases in Hamilton, Montana, developed the hamster model last year to mimic SARS-CoV-2 infection and mild disease in people. The University of Plymouth in the United Kingdom collaborated on these most recent studies.
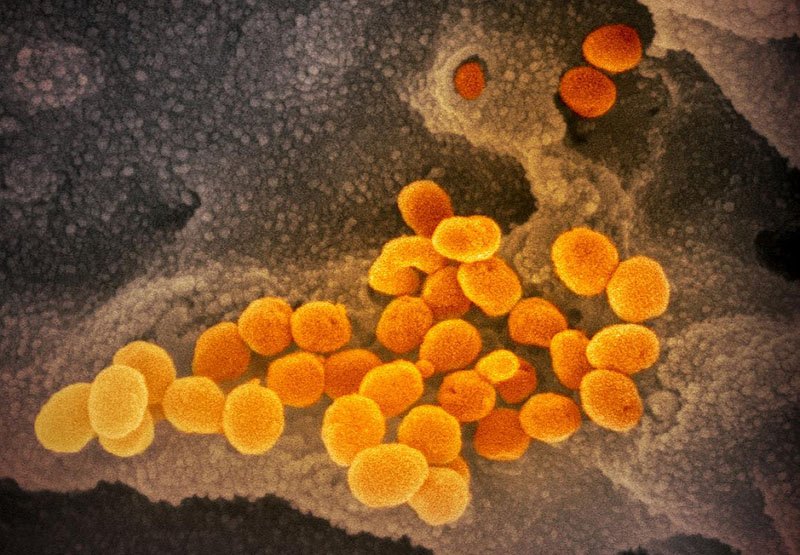
This scanning electron microscope image shows SARS-CoV-2 (orange)—also known as 2019-nCoV, the virus that causes COVID-19—isolated from a patient in the U.S., emerging from the surface of cells (gray) cultured in the lab.
This page was last updated on Friday, January 21, 2022