Antibody infusions prevent acquisition of some HIV strains, IRP studies find
Results will inform development of long-acting antibody-based HIV prevention tools
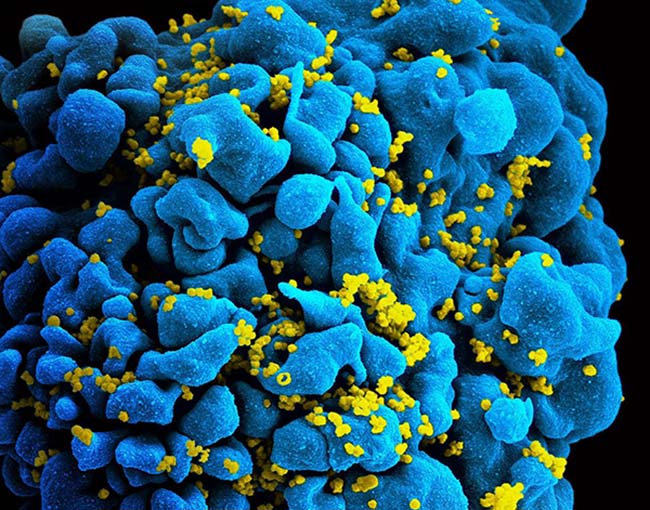
An investigational anti-HIV antibody delivered intravenously once every eight weeks safely and effectively prevented acquisition of HIV strains sensitive to that antibody, but did not significantly reduce overall HIV acquisition after 80 weeks among participants in two multinational clinical trials. Known as the Antibody-Mediated Prevention (AMP) Studies, the Phase 2b trials are sponsored and funded by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health. The studies are being conducted jointly by the HIV Vaccine Trials Network (HVTN) and HIV Prevention Trials Network (HPTN).
While currently available HIV treatment and prevention tools have greatly reduced transmission of the virus, there remains a need for long-acting HIV prevention strategies that are acceptable and desirable to people from diverse communities worldwide. Broadly neutralizing antibodies (bNAbs), which arise naturally in some people living with HIV and can stop a wide range of HIV strains from infecting human cells in the laboratory, are considered promising candidates for long-acting HIV prevention. These antibodies could be given directly by either infusion or injection or could be elicited by an HIV vaccine.
Launched in 2016, the AMP Studies aimed to establish whether infusions of a bNAb called VRC01 are safe, tolerable and effective at preventing HIV acquisition. The NIAID Vaccine Research Center (VRC) discovered VRC01 in 2010 in the blood of a person living with HIV and subsequently manufactured the antibody for the AMP Studies. The two trials included more than 4,600 participants. Men and transgender people who have sex with men were enrolled in the Americas and Europe — geographic regions where HIV subtype B predominates. Women were enrolled in sub-Saharan Africa, where HIV subtype C is dominant. Results will be discussed at a press conference and oral presentation during the 4th HIV Research for Prevention Conference.
This page was last updated on Friday, January 21, 2022