Vision to Grow as Scientist and Mentor
Kapil Bharti grows RPE from stem cells, aiming to restore damaged eyesight.

The team
A self-confessed bench scientist, Kapil Bharti, Ph.D., trained in molecular and developmental biology and eventually found his way to experimenting with new techniques for repairing the eye’s retinal pigment epithelium, or RPE. Now, he’s looking ahead to a Phase 1 clinical trial that could bring his dreams closer to a therapeutic reality for patients with RPE disorders.
“The RPE,” Bharti explains, “is a big sheet of cells in the back of the eye that do all the housekeeping jobs for photoreceptor cells, which are really the business end of the retina.”
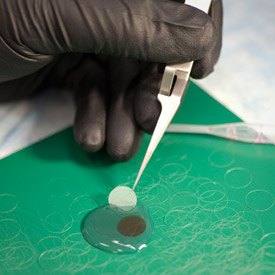
Kapil Bharti’s lab grows retinal pigment epithelium (RPE) cells on a three-dimensional biodegradable scaffold in preparation for clinical implantation trials.
Several eye diseases result from dysfunctional or damaged RPE cells, including age-related macular degeneration (AMD) and other degenerative retinal conditions, spurring Bharti to become an expert in the fundamentals of RPE biology. So it was no surprise in 2009 when, after a slew of papers demonstrated how to create RPE cells from induced pluripotent stem (iPS) cells, Bharti started to tinker in the same arena, aiming to improve the protocol and create the perfect RPE cell.
So, what makes an RPE cell perfect? Its shape? The pigment contained within? What are the functional and physiological traits that determine whether a cell is an RPE cell?

Sravya Kommineni, Fang Hua, and Ruchi Sharma (left to right) examine iPS cells, about 95 percent of which have become RPE cells.
Bharti’s group created a checklist of functions that a cell needs in order to be considered a true RPE cell, and, using a very strict set of criteria—including functional outputs (such as electrical resistance), physiological outputs (e.g., water transport), and genetic outputs (e.g., expression of certain genes)—they set off to investigate how accurately they could recreate a working RPE cell.
“It turns out that, over a period of eight to nine weeks, we can make committed human RPE cells from iPS progenitors, derived from human adult skin and blood, with about 95 percent efficiency,” Bharti says.

Mentoring early-career scientists is a primary focus of Bharti’s work in the lab: (left to right) Mostafa Lotfi, Kapil Bharti, Kiyoharu Josh Miyagishima, and Congxiao Zhang.
A key breakthrough in the research was Bharti’s development of a technique to grow the cells on a three-dimensional biodegradable scaffold, which allows the cells to mature in a more natural environment. The result is a three-dimensional sheet of fully functional, task-specific, human cells—functioning human tissue in most respects. For years, Bharti had been thinking about the potential to use his lab-grown RPE to treat degenerative eye diseases. But, how would a committed bench-scientist bring petri dishes of cells to a patient in need?

Bharti uses a hand-made model to explain eye development and the role of the RPE.
In early 2013, the NIH Common Fund announced a challenge to help basic scientists develop their research into treatments. Bharti and his lab were awarded a peer-reviewed grant from the program to enable them to move their iPS cell-derived RPE cells into a Phase 1 clinical study involving AMD patients over the next four years.
Unlike lab work, which is familiar enough to Bharti’s team, the translational step has been a giant leap into the unknown. Thankfully, a long list of collaborators and vendors are ready to supply skill sets, such as clinical-grade manufacturing and clinical research.

Vladimir Khristov (left) and Arvydas Maminishkis examine RPE cells grown on scaffolds to determine if the cells look and behave like native RPE.
Bharti leads the only team in the U.S. to be at such an advanced stage of development, although they are not the only researchers looking at the possibilities of bringing induced RPE cells into the clinic.
“That’s a good thing as far as I’m concerned,” Bharti says. “I’ve already approached many of these groups to help with our project, either through direct collaboration or through participation on our advisory committee. The more we collaborate, the faster we can all push the field forward.”
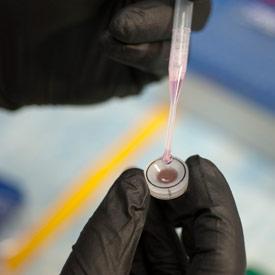
Compared to cells grown in media, RPE cells grown on biodegradable scaffolds develop more like in vivo RPE cells; Bharti’s team then tests the new cells to ensure correct electrophysiological characteristics.
While some translational research groups encourage an aura of secrecy around intellectual property, Bharti has embraced openness in leading the new venture, pitching his ideas and explaining novel techniques developed by his team that they believe will revolutionize the medical industry. He encourages collaboration in a pre-competitive space where multiple groups share resources, even while undertaking their own clinical trials. For instance, all the clinical-grade iPS cell lines made by Bharti’s group will be openly available to anyone who wishes to use them in further research.

Omar Memon (left) and Juliet Hartford prepare RPE cells for maturation as a single cell layer.
Despite managing a multi-million dollar research enterprise, with all the accompanying stresses, worries, and responsibilities, Bharti’s excitement seems to easily override any fears.
“It’s incredible to think that the most fundamental basic science discoveries in my lifetime will touch a patient,” Bharti says. “I think that, for a scientist, there cannot be a bigger satisfaction than this.”
Kapil Bharti, Ph.D., is a Stadtman Investigator in the Unit on Ocular & Stem Cell Translational Research at the National Eye Institute (NEI).
This page was last updated on Wednesday, May 24, 2023