Testing with combined biopsy method improves prostate cancer diagnosis in NIH study
Improved diagnosis could reduce the risk of both overtreatment and undertreatment of the disease
A method of testing for prostate cancer developed at the National Cancer Institute (NCI) leads to more accurate diagnosis and prediction of the course of the disease, according to a large study. This method, which combines systematic biopsy, the current primary diagnostic approach, with MRI-targeted biopsy, is poised to greatly improve prostate cancer diagnosis, thereby reducing the risk of both overtreatment and undertreatment of the disease. NCI is part of the National Institutes of Health.
The findings were published March 5, 2020, in the New England Journal of Medicine. The study was conducted at the NIH Clinical Center in Bethesda, Maryland.
“Prostate cancer has been one of the only solid tumors diagnosed by performing systematic biopsies ‘blind’ to the cancer’s location. For decades this has led to the overdiagnosis and subsequent unnecessary treatment of non-lethal cancers, as well as to missing aggressive high-grade cancers and their opportunity for cure,” said Peter Pinto, M.D., of the Urologic Oncology Branch in NCI’s Center for Cancer Research and senior author of the study. “With the addition of MRI-targeted biopsy to systematic biopsy, we can now identify the most lethal cancers within the prostate earlier, providing patients the potential for better treatment before the cancers spread.”
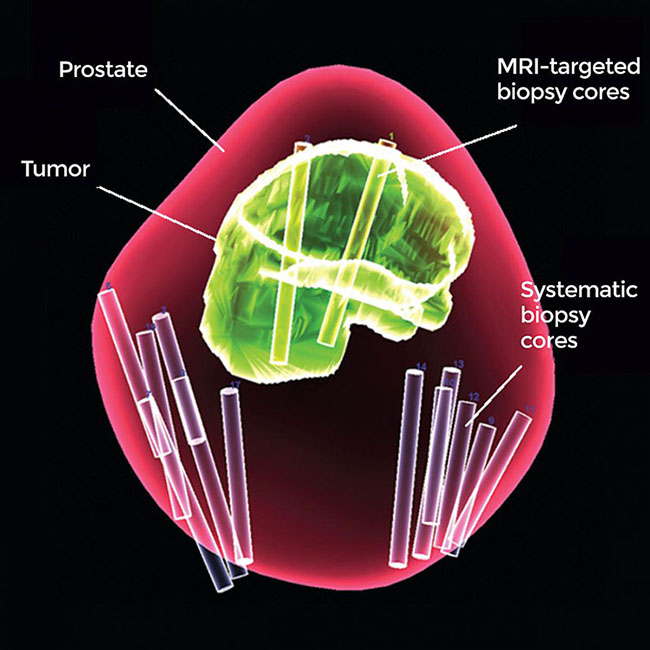
A 3-D map of the prostate using combined MRI-targeted and systematic biopsies. Using both types of biopsy greatly improved prostate cancer diagnosis in a new study.
This page was last updated on Friday, January 21, 2022