Researchers use patients’ cells to test gene therapy for rare eye disease
Approach could provide new path for difficult-to-treat forms of Leber congenital amaurosis
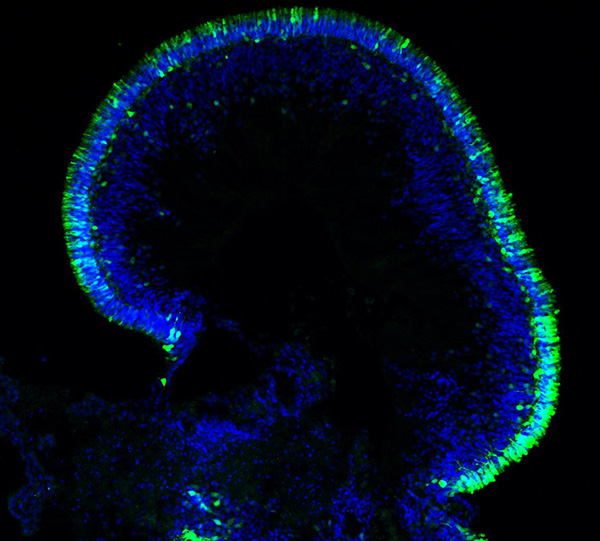
Scientists at the National Eye Institute (NEI) have developed a promising gene therapy strategy for a rare disease that causes severe vision loss in childhood. A form of Leber congenital amaurosis, the disease is caused by autosomal-dominant mutations in the CRX gene, which are challenging to treat with gene therapy. The scientists tested their approach using lab-made retinal tissues built from patient cells, called retinal organoids. This approach, which involved adding copies of the normal gene under its native control mechanism, partially restored CRX function. The study report appears today in Stem Cell Reports. NEI is part of the National Institutes of Health.
“Our treatment approach, which adds more copies of the normal gene, could potentially treat autosomal-dominant LCA caused by a variety of mutations,” said Anand Swaroop, Ph.D., chief of the NEI Neurobiology, Neurodegeneration and Repair Laboratory and senior author of the report.
The U.S. Food and Drug Administration approved Luxturna in 2017 for the treatment of LCA patients with mutations in a gene called RPE65. Although hailed as a major advance in gene therapy, Luxturna is ineffective against other forms of LCA, including those caused by autosomal-dominant mutations in CRX.
This page was last updated on Friday, January 21, 2022