First U.S. patient receives autologous stem cell therapy to treat dry AMD
At the National Institutes of Health, a surgical team successfully implanted a patch of tissue made from patient cells with the goal of treating advanced “dry” age-related macular degeneration (AMD), also known as geographic atrophy. Dry AMD is a leading cause of vision loss among older Americans and currently has no treatment.
The patient received the therapy as part of a clinical trial that is the first in the United States to use replacement tissues from patient-derived induced pluripotent stem (iPS) cells. The surgery was performed by Amir H. Kashani, M.D., Ph.D., associate professor of ophthalmology, Wilmer Eye Institute, Johns Hopkins School of Medicine with assistance by Shilpa Kodati, M.D., staff clinician, NEI. The procedure was performed at the NIH Clinical Center in Bethesda, Maryland, under a phase 1/2a clinical trial to determine the therapy’s safety.
This iPS cell derived therapy was developed by the Ocular and Stem Cell Translational Research Section team led by Kapil Bharti, Ph.D., senior investigator at the National Eye Institute (NEI), part of NIH, in collaboration with FUJIFILM Cellular Dynamics Inc., and Opsis Therapeutics, based in Madison, Wisconsin. Safety and efficacy of this cell therapy was tested by the NEI preclinical team. Clinical-grade manufacturing of this cell therapy was performed at the Center for Cellular Engineering, Department of Transfusion Medicine, Clinical Center, NIH.
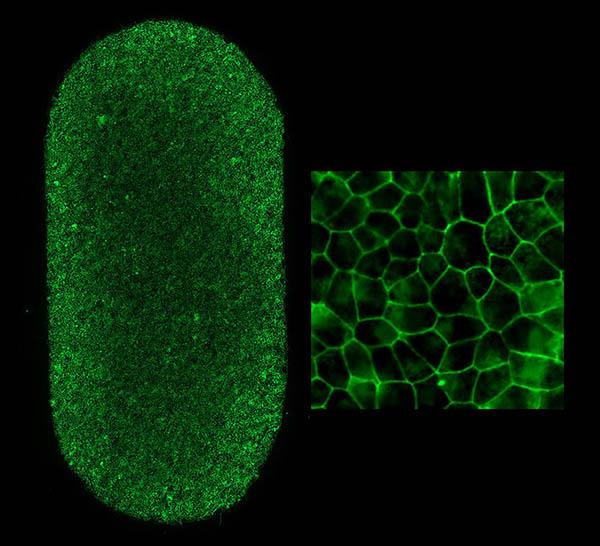
Left shows an image of the full-RPE-patch (2 x 4 mm). Each dot is an RPE cell with the borders stained green. Each patch contains approximately 75,000 RPE cells. Right image shows patch RPE cells at higher magnification.
This page was last updated on Tuesday, September 20, 2022