The Microbiome: When Good Bugs Go Bad
Yasmine Belkaid explores the fine balance that exists between microbes and their hosts.

The team
When Yasmine Belkaid, Ph.D., looks you firmly in the eye and says that every surface of your body is covered with thousands of different types of microbes, it’s hard not to recoil slightly. Our current hygiene-obsessed culture has a tough time accepting that our skin, gut, lungs, and even eyeballs are absolutely teeming with various mixes of microbiota: bacteria, fungi, and viruses.
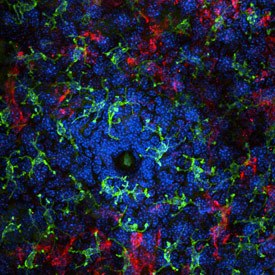
Immunofluorescent image of immune cells surrounding a hair follicle, enriched in commensal bacteria
“In terms of evolution, these microbes have always been with us,” Belkaid says. “Our bodily surfaces are constantly colonized by a microbiota, because we are ‘home’ for these microbes and they protect us as much as we protect them.”
Beneficial microbiota, termed “commensal” (meaning “to share the table”), compete for space on our body, thus stopping pathogenic microbes from setting up home. We have known of their existence for a long time, yet only recently have begun to understand how powerfully these microbes influence human physiology and wellbeing.

Research fellow Elizabeth Wohlfert (front) loads a cell sorter with samples, while Ph.D. student Michael Askenase observes cells under a microscope
Belkaid’s team studies the role of the microbiota in promoting immunity to infection, both in the gastrointestinal tract and on the skin, using an animal model that is completely free of any microbes. These mice live in a specially designed building on the IRP campus called a gnotobiotic facility. Born via cesarean section, they spend their entire lives in a completely germ-free environment, as if in a bubble. The animals are healthy, with a full and functioning immune system, but they have never been exposed to bacteria, viruses, or fungi of any kind.

A gnotobiotic (germ-free) mouse facility allows researchers to study how individual strains of bacteria influence the immune system
“It’s like starting at point zero,” Belkaid explains, “allowing us to selectively add back one, two, three, five, ten individual bacteria at a time—and then see what happens in terms of the immune response and, more explicitly, in terms of inflammation. This allows us for the first time, to show the link between defined bacteria and immune or inflammatory outcomes.”
Belkaid’s group showed that microbiota are essential to ensuring immunity against infection in both the skin and gut. Commensal bacteria stimulate the innate cells of our immune system to produce cytokines that fight invading microbes. Cytokines can also induce inflammation, which prompted Belkaid to query if imbalance of the microbiota could lead to disease.
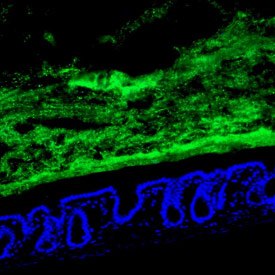
Healthy cells of the small intestine (blue) with commensal bacteria held at bay (green)
In general, diseases are a sign of imbalance and often caused by too strong an immune response. Some, like Crohn’s Disease and psoriasis, are caused by many factors, including genetics, but research now suggests that shifts in the microbiota can also contribute. For example, when the skin of someone with psoriasis is monitored there is a distinct shift in the microbiota compared to healthy skin.

Postdoctoral fellow Michael Molloy (front) trains high school student Josie Striepen in microscopy technique
The inflammation associated with psoriasis or Crohn’s disease has been well characterized, but Belkaid and her research team wanted to understand if that inflammatory response was partly due to an imbalance of the normal microbiota. Using the gnotobiotic animal model, they showed that some members of the microbiota are capable of inducing IL-17 from immune cells, an inflammatory cytokine implicated in the pathogenesis of psoriasis. Similarly her group explores how defined member of the gut microbiota could promote inflammatory responses. Interestingly, psoriasis and Crohn’s Disease occur primarily in the developed world. What changed in those societies and their environments to have such dramatic effects on people’s microbial health?
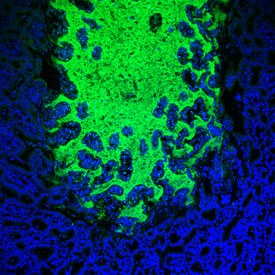
Commensal bacteria (green) invade the surface of the small intestine (blue) during parasite infection
“We may have altered many factors,” Belkaid says. “We’ve certainly changed our relationship with healthy microbiota by overusing antibiotics or changing our diet, but there are other considerations. For example, we used to live with parasitic worm infections, and in many ways this had a positive effect by preventing aberrant responses to allergens—self as well as our commensal bacteria. We are today in a state of imbalance with regards to our microbiota, and our response to that dysregulation has contributed to increased incidence of diseases such as allergies, inflammatory or autoimmune disorders.”

Dr. Belkaid discusses a research project with Ph.D. student Sean Spencer
The next step in Belkaid’s work aims to be translational. Can we alter the course of a disease by actively modifying human microbiota? Belkaid’s proximity to the NIH Clinical Center helps move her research forward by providing access to patients with inflammatory diseases, scientific collaborators who provide input and insight, and veterinarians who can identify disease symptoms in animal models. The result is a very efficient research process that works to keeps Belkaid’s team at the forefront of an exciting new field.
Yasmine Belkaid, Ph.D., is a Senior Investigator and Chief of the Mucosal Immunology Section in the Laboratory of Parasitic Diseases at the National Institute of Allergy and Infectious Diseases (NIAID).
This page was last updated on Thursday, January 4, 2024