Found in Translation
Bibiana Bielekova takes her work from the bench to the bedside and back again to discover a cure for multiple sclerosis.

When asked about the goals of her research, Bibiana Bielekova, M.D., doesn’t mince words: “We want to understand multiple sclerosis so that we can cure it.” A progressive neurodegenerative disease, multiple sclerosis presents several mysteries for researchers to solve. “We do know that the immune system plays a role in the disease,” explained Bielekova, “but we don’t know the targets in the brain or the mechanisms of attack.” Furthermore, the immune system is not simply the culprit in multiple sclerosis; inflammation also appears to play a role in repair of the nervous system.
Drugs like interferon-ß that dampen immune system function are currently used to treat multiple sclerosis. Bielekova believes that studying exactly how drugs are exerting their ameliorative effects in multiple sclerosis patients can lead to a better understanding of the disease process and a means of refining therapeutic strategies. Accordingly, Bielekova combines seeing patients and designing clinical trials with studying immune cells from patients in the laboratory. “If we can understand how a drug works on the molecular level and what it does on a functional level to inflammation of the brain and other symptoms, we can actually learn about the disease.”
Many of her studies have focused on an investigational drug for multiple sclerosis, daclizumab, that was first developed in the Intramural Research Program (IRP) by Thomas Waldmann, M.D., Ph.D. Daclizumab interferes with the function of an immune signaling molecule, IL-2. Initially designed to block immune rejection in transplant patients by targeting T cells that require IL-2 signaling, the drug was subsequently found to be an effective anti-inflammatory agent. In the course of this study, Bielekova’s team noticed an expanding population of unidentified cells in blood samples from their patients.
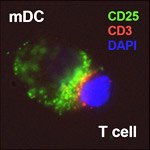
To solve this mystery, Bielekova conducted another identical trial and discovered that these cells were another population of immune cells, NK cells, that were killing off T cells. “We had a hard time publishing this observation, which went against dogma, but since then other researchers found that interferon-ß and other treatments that have beneficial effects in multiple sclerosis also cause an expansion or activation of this same population of cells.” Increasingly, evidence points to the role of NK cells in maintaining immune tolerance—the ability of the immune system to recognize self and only target foreign invaders—and combating multiple sclerosis.

Bielekova’s laboratory is conducting additional work to understand the cellular mechanisms of daclizumab’s clinical efficacy. Meanwhile, daclizumab has been licensed to a pharmaceutical company that is taking it through phase 3 clinical trials. “Our goal is that by the time the drug is approved by the FDA, we’ll know everything about it—how it really works, what the good effects and side effects are, and who will best respond to the treatment.”
The issue of selecting the right drugs for the right patients is important to Bielekova. “When I see a patient in a clinic, I’d like to say that he or she has this type of immune system, this type of disease, and so I will prescribe this drug,” said Bielekova. “Currently, there are eight multiple sclerosis drugs on the market, and we only have a trial-and-error approach to treatment.” To improve these odds, she leads a natural history study in which patients are referred to the NIH by their primary neurologists. “They have a world-class work-up to inform the diagnosis and treatment of their disease; we have biological samples to inform our understanding of the disease over time;” a win-win situation for patients, doctors and researchers.
Bibiana Bielekova, M.D., is an Investigator in the Neuroimmunological Diseases Unit of the National institute of Neurological Disorders and Stroke (NINDS).
Further reading:
Research studies actively recruiting multiple sclerosis patients:
This page was last updated on Wednesday, May 24, 2023