Changing Cells, Aging Bodies
Myriam Gorospe views aging through a molecular lens to help lengthen healthspan.

Ji-Heon Noh, Ph.D. (left), and Myriam Gorospe, Ph.D., discuss the selection of mitochondrial RNA samples in an experiment to study energy generation by senescent cells
Each moment of life, the environment contributes to changes in our bodies at the cellular and molecular levels that can greatly affect our overall health. From toxins and nutrients to natural limits on cellular replication, Myriam Gorospe, Ph.D., and her team have dedicated nearly 15 years to studying the fundamental mechanisms of growing older.
Considering the range of effects that aging has on the body and its myriad organs, tissues, and cells, understanding why we all don’t live healthy and happy forever is no simple feat.
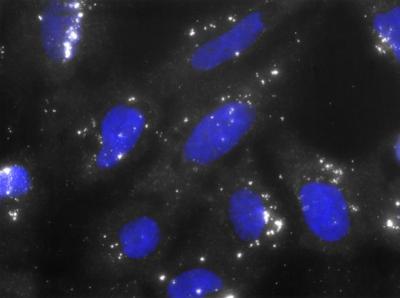
FISH (fluorescence in situ hybridization) of the senescence-associated lncRNA HOTAIR in human diploid fibroblasts
“The study of aging could be considered daunting since it’s so broad,” Gorospe says. “On the other hand, it means we have a wide canvas on which to examine the questions we’re most interested in. In our lab, we approach the study of aging from the perspective of the cell, focusing on a range of aging processes – some of which are pathological, but many of which are simply physiological and just as poorly understood.”

Kotb Abdelmohsen, Ph.D., prepares radioactive DNA probes to investigate the abundance of long noncoding RNAs in senescent cells using Northern blot analysis
One of Gorospe team’s research focus areas is cellular senescence, a natural state of permanent cell cycle arrest reached when cells stop dividing, usually after 50 or so divisions. Cellular senescence was discovered four decades ago, but scientists still don’t fully understand why it happens. One of the most widely accepted explanations is that the ends of each cell’s chromosomes—called telomeres—shorten a little during each replication and at some point signal the cell to stop dividing in order to protect itself from potential damage. The cells don’t necessarily die as a result, but they can no longer divide and function like younger, healthy cells.

Myriam Gorospe, Ph.D., Amaresh Panda, Ph.D. (center) and Rong Guo, Ph.D. (right) plan next experiments involving RNA sequencing
“As we age, along with the reduced capacity to divide, our cells progressively lose their ability to function optimally, and these changes are often associated with development of age-related diseases,” Gorospe says. “One example that I think most people can relate to is diabetes. As we grow old, our body’s ability to quickly react to changing glucose levels in our bloodstream is gradually impaired. So, as we age into our 50s, 60s, and 70s, we start to see more and more diabetes. One of the questions my group studies is why this decline happens.”
Cultured muscle cells are used to investigate the process of muscle differentiation (myogenesis), which is impaired with advancing age. Studies are underway to investigate age-associated RNA-binding proteins and long noncoding RNAs that influence myogenesis
To gain further insight into this question, a former researcher in Gorospe’s lab, Eun Kyung Lee, Ph.D., studied the responses of cultured pancreatic beta cells to high levels of glucose in the culture medium. The team discovered that in response to high glucose, the RNA-binding protein HuD was released from the insulin mRNA, allowing the insulin mRNA to be made into protein and causing insulin levels to rise. When the group further investigated insulin levels in animals that overexpressed the HuD gene, they found markedly reduced levels of insulin, suggesting that abnormally high HuD levels or function could underlie some cases of age-related diabetes.

Protein concentration, as measured using the Bradford assay
Moving this research forward will require collaboration, including clinical investigation with patients who have developed age-related diabetes—a challenging task for many basic science laboratories to consider—yet Gorospe is excited at the prospect of translating their findings from the bench to the bedside.

Dr. Gorospe and Je-Hyun Yoon, Ph.D., examine neurons using fluorescence microscopy; here, RNA-binding proteins are investigated in order to understand the molecular basis of Alzheimer’s disease
“It’s gratifying to work in an environment where we’re allowed to focus on our area of expertise, but very much encouraged to collaborate across disciplines so that we can study these questions holistically,” Gorospe says. “In our lab, we focus on molecular and cellular biology, but when we have questions that require additional resources or expertise, technical or biological, we simply go to our collaborators downstairs or a few doors away. Because our resources are shared, there is no real competition. We’re all working together towards a common goal. In my mind, with this approach, we’re much more likely to find the answers to aging as healthily as possible that we’re all ultimately seeking.”
Myriam Gorospe, Ph.D., is a Senior Investigator and Chief of the RNA Regulation Section in the Laboratory of Genetics at the National Institute on Aging.
This page was last updated on Wednesday, May 24, 2023