Cellular Trash-Talking
Rosa Puertollano dives into the mechanisms of cellular cleanup.

Postdoctoral fellows Heba Diab (left) and Lu Sun (right) prepare cells for analysis and discuss their research project with team leader Rosa Puertollano
Take flight through the inside of a cell—be it hepatocyte, myocyte, or neuron—and you might recognize the nucleus as the control hub of cellular activity, mitochondria, the cell’s energy production centers; and the endoplasmic reticulum, site of protein synthesis. Less recognizable, yet critical to the function of all cells, are the lysosomes: masters of cellular waste removal and recycling.
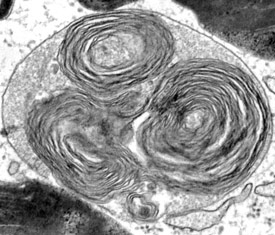
Defective lysosomal function in Mucolipidosis Type IV patients results in accumulation of undegraded material inside lysosomes
In her research lab at the NIH, Rosa Puertollano, Ph.D., works at the center of a resurging interest in lysosomes that is changing how people view biological processes from energy production to aging. Despite the perhaps unflattering comparison to a garbage disposal, lysosomes are crucial to keeping our cells healthy. Defects in lysosome function can lead to an accumulation of cellular debris, now known to cause more than 70 human diseases.
“These are very basic and very important organelles that were actually discovered in the 1960s, but the research community forgot about them,” Puertollano explains. “Now, people are starting to explore the lysosome in more detail, because it’s become apparent that they do so much more than people at first imagined.”

Huiqing Li, a postdoctoral fellow, transfers juvenile zebrafish to a dish for examination of their development
The list of household chores that the lysosome undertakes includes nutrient recycling—processing used materials back into the molecular building blocks of the cell—and clearing pathogens from the cell. For example, when immune cells “eat,” or phagocytose, bacteria, their lysosomes remove the byproducts. Lysosomes have an additional function that, while radical in concept, is essential to maintaining good tissue health: cannibalizing other organelles to keep the whole cell alive when nutrients are low, a process known as autophagy.

Zebrafish offer a uniquely useful model of cellular development, thanks to their transparent bodies and sharing many biological pathways with humans
“One of our newest findings in this field is that lysosomes play a critical role in nutrient sensing,” Puertollano says. “When you have plenty of food, you can grow and proliferate. But if you don’t have food, you have to stop growing and activate stress pathways that will tell a cell how to respond. We now know that one of the most important components of this signaling pathway is a protein kinase called mTOR, a decision-making molecule activated on the surface of the lysosome that can determine the amount of nutrients available.”
A juvenile zebrafish swims under the microscope—the Puertollano group uses zebrafish to understand lysosomal disease
Puertollano’s group found that mTOR regulates the activation of TFEB and TFE3, two transcription factors that promote the formation of lysosomes, as well as autophagy induction. The discovery revealed that cells regulate lysosomal function in response to nutrient levels and changing environmental conditions. Additionally, over-expressions of TFEB or TFE3 alert the cell to send its lysosomes to the cell membrane, where lysosomes release their contents in a massive cleanup of the cell.

Dr. Sun uses flow cytometry to analyze mouse cells and address the physiological function of specific lysosomal proteins in vivo
Current treatments for lysosomal diseases are challenging, and many remain life-threatening. Puertollano and colleagues wondered if, no matter what the underlying pathology, they could convince a cell to fuse its lysosomes with the plasma membrane—essentially telling the cell that it’s garbage day, even when it’s not—which would perhaps clear any toxic buildup of proteins within the lysosomes.
With a viral vector that overexpresses the TFE3 transcription factor, Puertollano and collaborators tested their hypothesis in the “Pompe mouse,” an animal model of Pompe disease, the rare, inherited progressive disorder caused by accumulation of glycogen in the lysosomes that eventually destroys muscle, leading to paralysis and heart failure.

Jose Martina, a staff scientist in Dr. Puertollano’s lab, looks at fluorescently labeled lysosomes to determine the mTOR-dependent regulation of TFEB
In collaboration with the group of Nina Raben, the team found that overexpression of TFE3 results in a dramatic reduction of the number of glycogen-filled lysosomes in cultured muscle cells from the Pompe mouse.

The team led by Dr. Puertollano seeks new ways of understanding how lysosomes function in health and disease
“We need to be cautious with our next steps, as we don’t yet know what other effects could result from this type of therapy,” Puertollano says, “but it’s hard not to get excited about this approach, because there are so many other lysosomal storage diseases in which this might work—Gaucher Disease, Niemann-Pick Disease, Tay-Sachs, and many more.”
Puertollano’s lab is now testing a vector-based over-expression system in cells from an array of animal models that replicate rare lysosomal storage diseases, with the hope that there may a path to therapy from a better understanding of the “lowly” lysosome.
Rosa Puertollano is a Senior investigator in the Cell Biology and Physiology Center at the National Heart, Lung, and Blood Institute (NHLBI).
This page was last updated on Wednesday, May 24, 2023