A Conviction, an Addiction, and a Mouse
Veronica Alvarez takes the path less traveled to understand addiction.

Principal Investigator Veronica Alvarez (center) at work in the lab with postdoctoral fellows Martín Adrover (left) and Jung Hoon Shin
In the office next to her lab, Veronica Alvarez, Ph.D., jokes that an afternoon diet cola helps her get through the day. Then, becoming much more serious, she acknowledges that addiction can be a chronic disease that destroys families, careers, and lives. Who is at risk for addiction and how do genetics affect the risk and severity of disease are questions that Alvarez approaches from an unusual angle.
“I arrived at the IRP with a desire to better understand the factors that influence addiction,” Alvarez says, “and I believed that genetically engineered mice could help.”

Martín Adrover measures dopamine release from neurons in live brain tissue under a microscope using an electrochemical technique called cyclic voltammetry
Mice with a gene of interest that has been deactivated—or “knocked out”—have assisted researchers in learning about human disease for decades, but only recently are they being used to examine addictive behaviors.
“That’s something that perhaps only a handful of labs in the US are currently doing,” Alvarez explains. “Most of my colleagues use rats. But I was convinced that by modifying the mouse genetic background in very precise ways we could help answer some long-standing questions in the field of addiction.”
A central question relates to dopamine, an important neurotransmitter made and released by a set of neurons in the middle part of the brain. Science has shown a role for dopamine in drug addiction, but conflicting evidence on the type of dopamine receptors involved, and which receptor-carrying neurons contribute to addictive behaviors, has hampered efforts at pharmacotherapy. So, armed with a miniaturized behavior box, the support of her colleagues in adjoining labs, and hope that the mice would cooperate, Alvarez began to investigate.

Postbaccalaureate fellow Katie Holroyd (left) and Dr. Alvarez analyze dendritic spines in medium spiny neurons in the nucleus accumbens of a mouse lacking dopamine autoreceptors
Collaborating with Marcelo Rubinstein’s group in Buenos Aires, the Alvarez team created a genetically altered mouse lacking only dopamine autoreceptors (dopamine receptors present on the same neurons that make and release dopamine). The animals—termed conditional knockouts—were physically identical to their littermates, but due to the genetic mutation they lacked the dopamine receptors that act as a “brake”—or inhibitory feedback—telling neurons to stop releasing dopamine when there is enough around.
The first experiments were challenging. Most of the team was new to behavioral science, but they persevered, designing an experimental protocol in which both the normal and the genetically altered mice received a cocaine reward when they poked their noses in a hole.
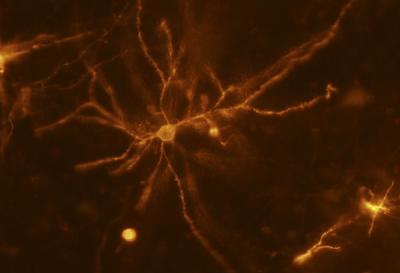
A mouse neuron in the striatum imaged using a two-photon optical microscope allows researchers to measure changes to dendritic spines and their influence on addiction
When the results were analyzed, not only did the dopamine-receptor knockout mice learn the task faster (in less than a week), but they were all also poking for the reward, whereas the wild-type mice learned more slowly, with one-third of them not learning the task at all.
The vulnerability of the transgenic mice to engage in cocaine taking was exacerbated by another trait of these animals that the group discovered when no longer providing cocaine, replicating drug withdrawal. When the mice did not receive cocaine after each poke, but instead only found cues that had been associated with the drug—like the drug paraphernalia for people—the wild-type mice stopped responding after a week or so. But those without dopamine autoreceptors continued to seek cocaine for as long as they were tested (about a month) and work hard just for a chance to get the drug-paired “signals,” such as colored lights that appear for a few seconds.

Postdoctoral fellow Julia Lemos uses suction to apply negative pressure that allows an electrode to fuse with a cell under a two-photon fluorescence microscope
The experiment joins a short but growing list of studies that clearly demonstrate to the research community that mice can advance behavioral science and investigators can address questions around complex human behavior in this way.
A question topping Alvarez’s list for further study is, what genetic factors influence different responses to drug signals and their role in addiction? For example, while we know that a sign for a “BAR” means that the establishment serves alcohol (the same meaning for all of us), individuals suffering from alcohol abuse or dependence are likely to be drawn to it and unable to resist the temptation to go inside and engage in drinking (a strong attractive response).
The team (l to r): Jillian Iafrati , Alanna Kaplan, Martín Adrover, Julia Lemos, Katie Holroyd, Veronica Alvarez, Jung Hoon Shin, Lauren Dobbs
It turns out that there is some innate variability among humans in their responses to signals that predict rewards. Some individuals show a strong attraction for not just rewards but also the signals that predict them (sign trackers), whereas others are mainly attracted to the reward itself (goal trackers). People suffering from addictive disorders tend to have the personality trait of sign trackers, perhaps due to a more intense dopamine release or the presence of fewer dopamine autoreceptors. Understanding differences like these will assist researchers pursue targeted therapies that may help people overcome addiction disorders.
Veronica Alvarez, Ph.D., is a Principal Investigator in the Section of Neuronal Structure of the Laboratory for Integrative Neuroscience at the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and also receives funding from the National Institute of Neurological Disorders and Stroke (NINDS).
This page was last updated on Wednesday, May 24, 2023